Abstract
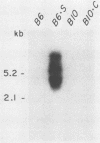
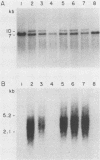
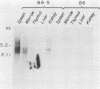
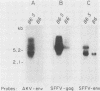
Expression of endogenous retroviral sequences in Fv-2 congenic mouse strains was examined by Northern blot analysis. Endogenous ecotropic virus transcripts were observed in total spleen RNA of B6.S (Fv-2ss) mice. Endogenous ecotropic transcripts were not detected in spleen RNA of C57BL/6, the Fv-2rr congenic partner of B6.S, nor in spleens of the C57BL/10 (Fv-2rr) and B10.C (Fv-2ss) congenic strains. Mendelian segregation analysis revealed that only backcross mice segregating the newly acquired Fv-2-linked endogenous ecotropic provirus had endogenous ecotropic transcripts in spleen RNA. Examination of different tissues of B6.S mice showed that Emv-18 transcription was highest in spleen and bone marrow, tissues in which Fv-2 has been shown to function. These results support the conclusion that chromosomal location is an important factor controlling Emv-18 expression in B6.S mice. We also report the presence in the spleen of a novel xenotropic virus transcript detectable only in B6.S mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosselman R. A., Van Griensven L. J., Vogt M., Verma I. M. Genome organization of retroviruses IX. Analysis of the genomes of Friend spleen focus-forming (F-SFFV) and helper murine leukemia viruses by heteroduplex-formation. Virology. 1980 Apr 15;102(1):234–239. doi: 10.1016/0042-6822(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Fluidity of a retrovirus genome. J Virol. 1984 Jun;50(3):759–765. doi: 10.1128/jvi.50.3.759-765.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Lee B. K. Association of the lethal yellow (Ay) coat color mutation with an ecotropic murine leukemia virus genome. Proc Natl Acad Sci U S A. 1983 Jan;80(1):247–249. doi: 10.1073/pnas.80.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulari N. G. Murine leukemia viruses with recombinant env genes: a discussion of their role in leukemogenesis. Curr Top Microbiol Immunol. 1983;103:75–108. doi: 10.1007/978-3-642-68943-7_4. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Germ-line MuLV reintegrations in AKR/J mice. Nature. 1982 Apr 29;296(5860):865–868. doi: 10.1038/296865a0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon W. Y., Theodore T. S., Buckler C. E., Stimpfling J. H., Martin M. A., Morse H. C., 3rd Relationship between a retroviral germ line reintegration and a new mutation at the ashen locus in B10.F mice. Retroviral integration and an ashen mutation. Virology. 1984 Feb;133(1):183–190. doi: 10.1016/0042-6822(84)90437-9. [DOI] [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Mak T. W., Axelrad A. A., Bernstein A. Fv-2 locus controls expression of Friend spleen focus-forming virus-specific sequences in normal and infected mice. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5809–5812. doi: 10.1073/pnas.76.11.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey J., Risser R. Genetic interactions in the spontaneous production of endogenous murine leukemia virus in low leukemic mouse strains. J Exp Med. 1982 Aug 1;156(2):337–349. doi: 10.1084/jem.156.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll B., Hartley J. W., Rowe W. P. Induction of B-tropic and N-tropic murine leukemia virus from B10.BR/SgLi mouse embryo cell lines by 5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1979 Jul;63(1):213–217. [PubMed] [Google Scholar]
- Mowat M., Bernstein A. Linkage of the Fv-2 gene to a newly reinserted ecotropic retrovirus in Fv-2 congenic mice. J Virol. 1983 Sep;47(3):471–477. doi: 10.1128/jvi.47.3.471-477.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Risser R., Horowitz J. M., McCubrey J. Endogenous mouse leukemia viruses. Annu Rev Genet. 1983;17:85–121. doi: 10.1146/annurev.ge.17.120183.000505. [DOI] [PubMed] [Google Scholar]
- SNELL G. D., BUNKER H. P. HISTOCOMPATIBILITY GENES OF MICE. V. FIVE NEW HISTOCOMPATIBILITY LOCI IDENTIFIED BY CONGENIC RESISTANT LINES ON A C57B 10 BACKGROUND. Transplantation. 1965 Mar;3:235–252. doi: 10.1097/00007890-196503000-00011. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steffen D. L., Taylor B. A., Weinberg R. A. Continuing germ line integration of AKV proviruses during the breeding of AKR mice and derivative recombinant inbred strains. J Virol. 1982 Apr;42(1):165–175. doi: 10.1128/jvi.42.1.165-175.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]