Abstract
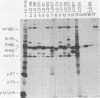
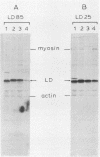
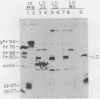
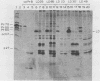
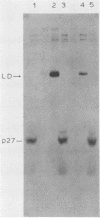
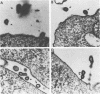
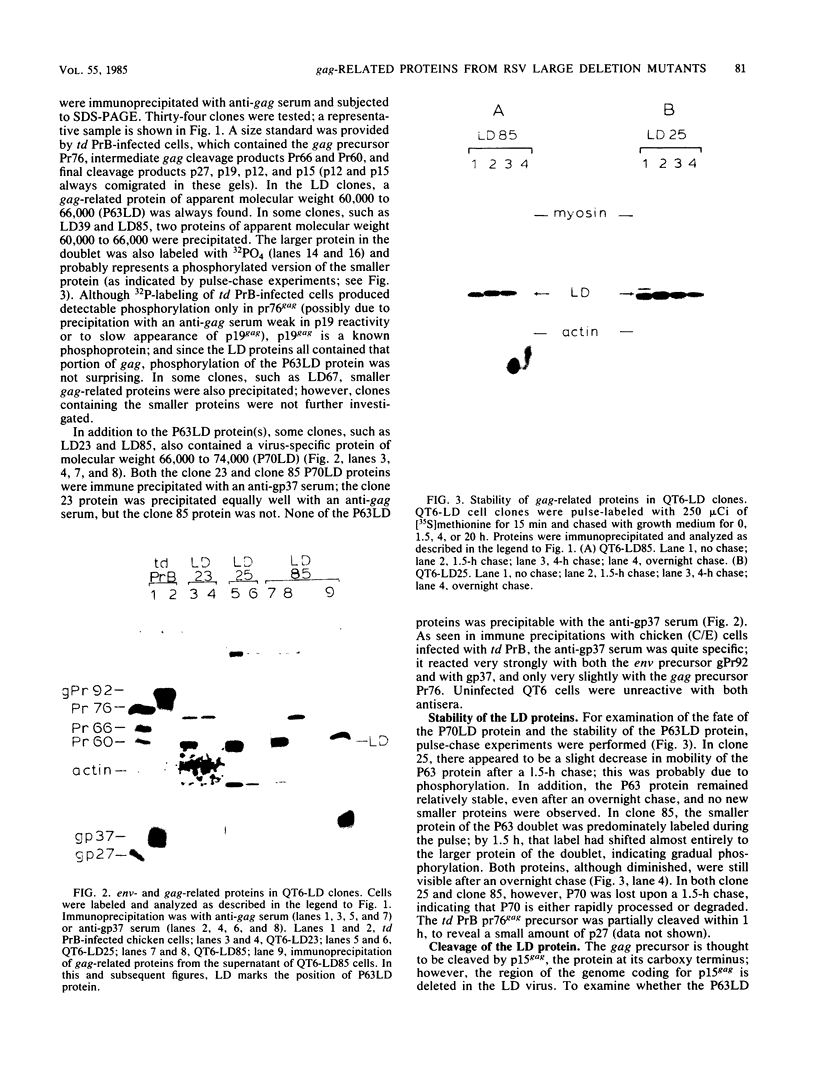
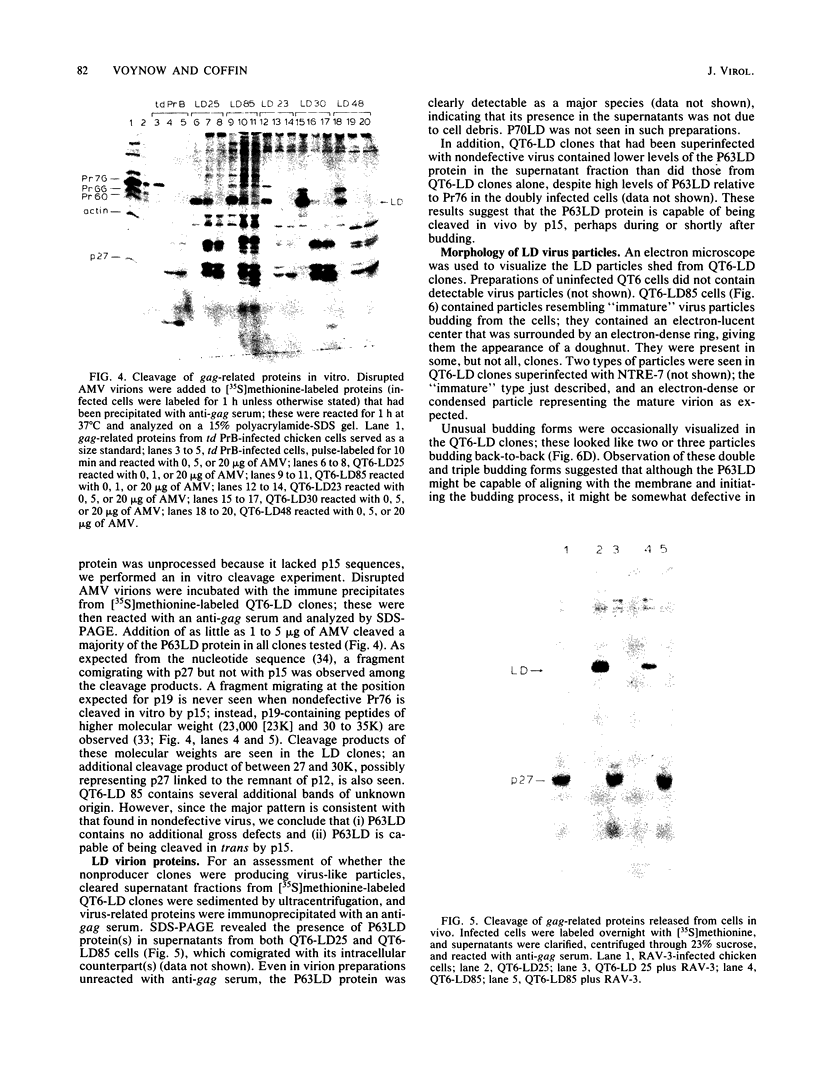
Large deletion (LD) mutants of Prague strain Rous sarcoma virus subgroup B (PrB), derived by serial undiluted passage through chicken (C/E) cells, contain two deletions relative to wild-type virus. One of these joins gag sequences in the p12 coding region to env sequences in region encoding gp37; the other deletion spans the src region. Analysis of the viral proteins of QT6 cell clones containing only LD proviruses by sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a major truncated gag-related phosphoprotein of 60,000 to 66,000 daltons (P63LD). P63LD was stable, but could be cleaved in vitro to the predicted products by p15gag. A second gag-related LD protein of about 68,000 to 74,000 molecular weight (P70LD) was also found which often reacted with an anti-gp37 serum. P70LD was unstable and may represent a short-lived gag-gp37 fusion protein. Finally, immunoprecipitation indicated that particles containing P63LD were shed from QT6-LD clones. Thin section preparations of these clones viewed in an electron microscope showed enveloped budding particles of "immature" morphology. Thus, the synthesis and release of particles from infected cells does not require cleavage of the gag precursor, nor does it require the presence of p15 or (most of) p12.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Robinson H. L. Gs, an allele of chickens for endogenous avian leukosis viral antigens, segregates with ev 3, a genetic locus that contains structural genes for virus. J Virol. 1979 Aug;31(2):420–425. doi: 10.1128/jvi.31.2.420-425.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P., Luftig R., Shaper J. H. Localization of RNA tumor virus polypeptides. I. Isolation of further virus substructures. Virology. 1973 Dec;56(2):549–564. doi: 10.1016/0042-6822(73)90057-3. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Tsichlis P. N., Barker C. S., Voynow S., Robinson H. L. Variation in avian retrovirus genomes. Ann N Y Acad Sci. 1980;354:410–425. doi: 10.1111/j.1749-6632.1980.tb27982.x. [DOI] [PubMed] [Google Scholar]
- Conklin K. F., Coffin J. M., Robinson H. L., Groudine M., Eisenman R. Role of methylation in the induced and spontaneous expression of the avian endogenous virus ev-1: DNA structure and gene products. Mol Cell Biol. 1982 Jun;2(6):638–652. doi: 10.1128/mcb.2.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R., Shaikh R., Mason W. S. Identification of an avian oncovirus polyprotein in uninfected chick cells. Cell. 1978 May;14(1):89–104. doi: 10.1016/0092-8674(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Gebhardt A., Bosch J. V., Ziemiecki A., Friis R. R. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984 Apr 5;174(2):297–317. doi: 10.1016/0022-2836(84)90340-1. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Kung H. J., Bailey J. M., Davidson N., Vogt P. K., Nicolson M. O., McAllister R. M. Electron microscope studies of tumor virus RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):827–834. doi: 10.1101/sqb.1974.039.01.096. [DOI] [PubMed] [Google Scholar]
- Lu A. H., Soong M. M., Wong P. K. Maturation of Moloney murine leukemia virus. Virology. 1979 Feb;93(1):269–274. doi: 10.1016/0042-6822(79)90297-6. [DOI] [PubMed] [Google Scholar]
- Mermer B., Malamy M., Coffin J. M. Rous sarcoma virus contains sequences which permit expression of the gag gene in Escherichia coli. Mol Cell Biol. 1983 Oct;3(10):1746–1758. doi: 10.1128/mcb.3.10.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montelaro R. C., Sullivan S. J., Bolognesi D. P. An analysis of type-C retrovirus polypeptides and their associations in the virion. Virology. 1978 Jan;84(1):19–31. doi: 10.1016/0042-6822(78)90215-5. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Frank H., Schäfer W. Properties of mouse leukemia viruses. 3. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze-drying and freeze-etching. Virology. 1972 Aug;49(2):345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Cappiello D., Wilkowski C., Vogt V. M. Chemical crosslinking of proteins in avian sarcoma and leukemia viruses. Virology. 1980 Apr 15;102(1):205–210. doi: 10.1016/0042-6822(80)90081-1. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Vogt V. M. Identification of retrovirus matrix proteins by lipid-protein cross-linking. J Mol Biol. 1979 Jul 15;131(4):819–837. doi: 10.1016/0022-2836(79)90203-1. [DOI] [PubMed] [Google Scholar]
- Pinter A., deHarven E. Protein composition of a defective murine sarcoma virus particle possessing the enveloped type-A morphology. Virology. 1979 Nov;99(1):103–110. doi: 10.1016/0042-6822(79)90041-2. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Anderson S. M., Riemen M. W., Hanafusa H. gag-Related polypeptides encoded by replication-defective avian oncoviruses. J Virol. 1979 Dec;32(3):749–761. doi: 10.1128/jvi.32.3.749-761.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of an infectious virus-antibody complex with Rous sarcoma virus and antibodies directed against the major virus glycoprotein. J Virol. 1976 Mar;17(3):1063–1067. doi: 10.1128/jvi.17.3.1063-1067.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Bolognesi D. P. Mammalian C-type oncornaviruses: relationships between viral structural and cell-surface antigens and their possible significance in immunological defense mechanisms. Contemp Top Immunobiol. 1977;6:127–167. doi: 10.1007/978-1-4684-3051-6_4. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. The genome-associated, specific RNA binding proteins of avian and mammalian type C viruses. Cell. 1977 Jan;10(1):91–99. doi: 10.1016/0092-8674(77)90143-x. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Pinter A. Interferon-mediated inhibition of production of Gazdar murine sarcoma virus, a retrovirus lacking env proteins and containing an uncleaved gag precursor. Virology. 1983 Apr 15;126(1):403–407. doi: 10.1016/0042-6822(83)90492-0. [DOI] [PubMed] [Google Scholar]
- Stromberg K., Hurley N. E., Davis N. L., Rueckert R. R., Fleissner E. Structural studies of avian myeloblastosis virus: comparison of polypeptides in virion and core component by dodecyl sulfate-polyacrylamide gel electrophoresis. J Virol. 1974 Feb;13(2):513–528. doi: 10.1128/jvi.13.2.513-528.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg K. Surface-active agents for isolation of the core component of avian myeloblastosis virus. J Virol. 1972 Apr;9(4):684–697. doi: 10.1128/jvi.9.4.684-697.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Bruckenstein D. A., Bell A. P. Avian sarcoma virus gag precursor polypeptide is not processed in mammalian cells. J Virol. 1982 Nov;44(2):725–730. doi: 10.1128/jvi.44.2.725-730.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Ishigame K., Ohno T., Kageyama S., Shibata K., Luftig R. B. Preparations enriched for "immature" murine leukemia virus particles that remain in tissue culture fluids are deficient in Pr65gag proteolyic activity. Virology. 1980 Jan 15;100(1):130–140. doi: 10.1016/0042-6822(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Morphological conversion of 'immature' Rauscher leukaemia virus cores to a 'mature' form after addition of the P65-70 (gag gene product) proteolytic factor. J Gen Virol. 1978 Jul;40(1):151–160. doi: 10.1099/0022-1317-40-1-151. [DOI] [PubMed] [Google Scholar]
- Yoshinaka Y., Luftig R. B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand ("immature") to a collapsed ("mature") form of the virus core. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]