Abstract
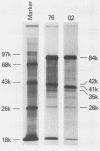
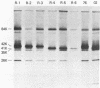
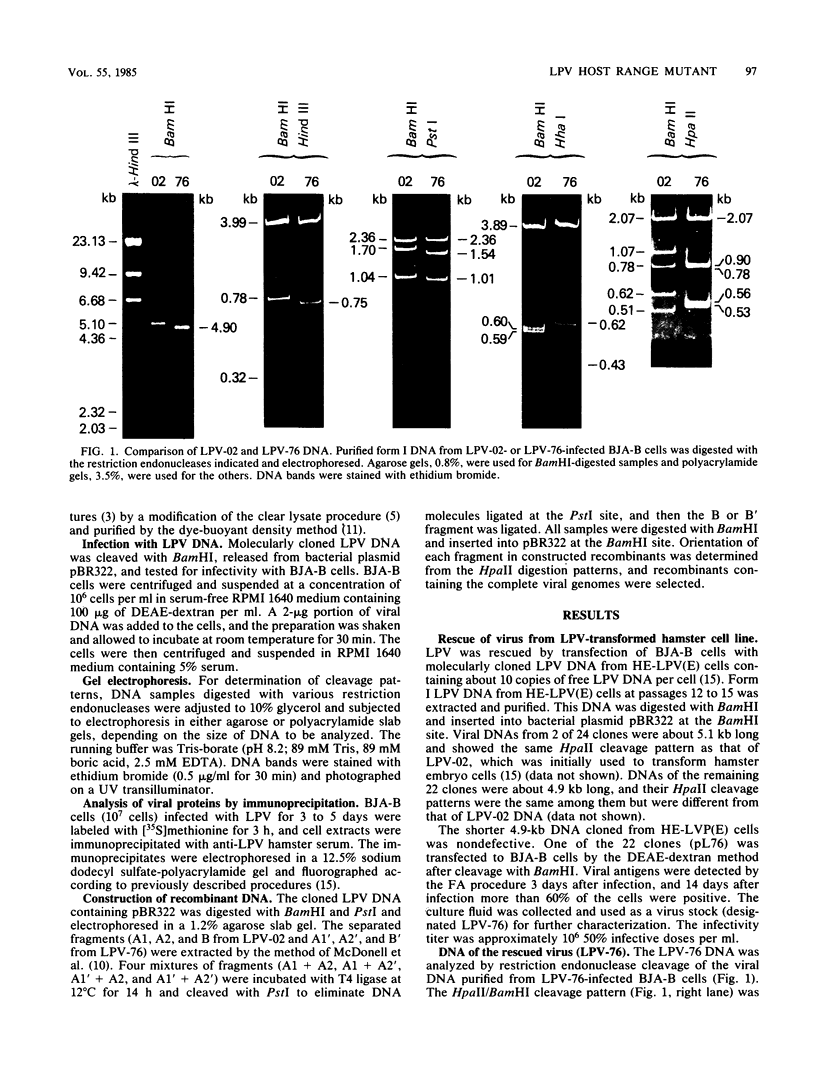
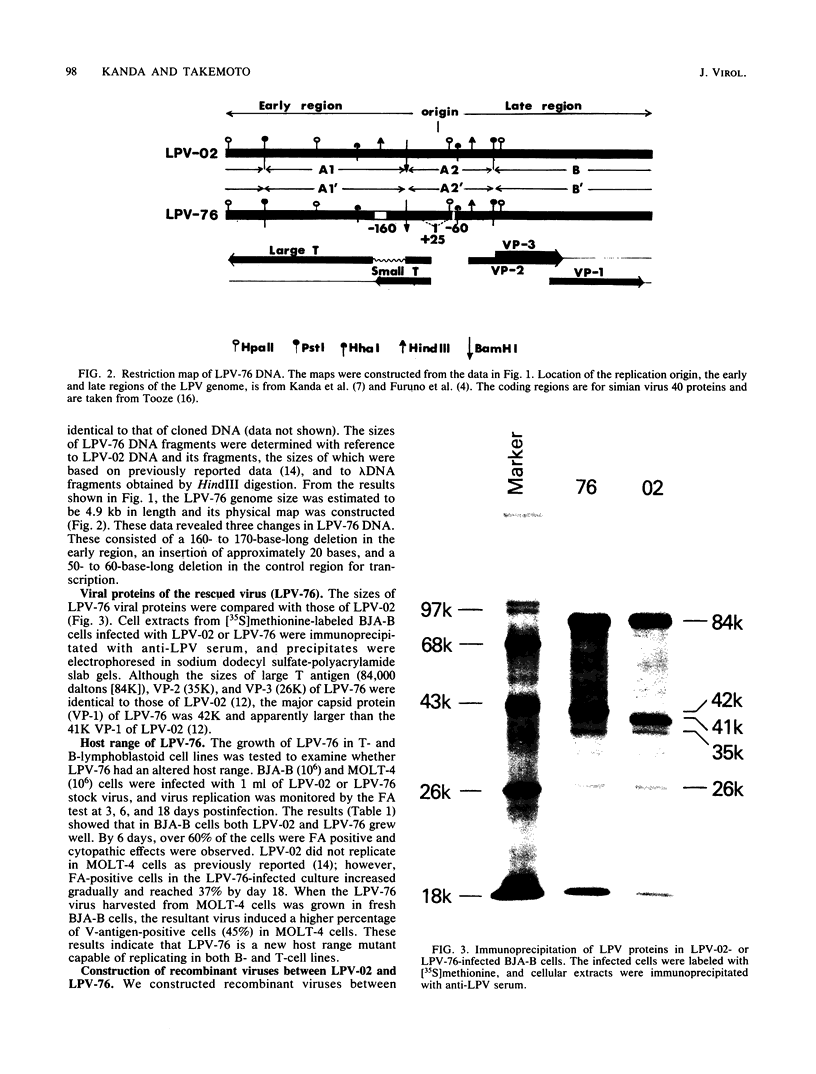
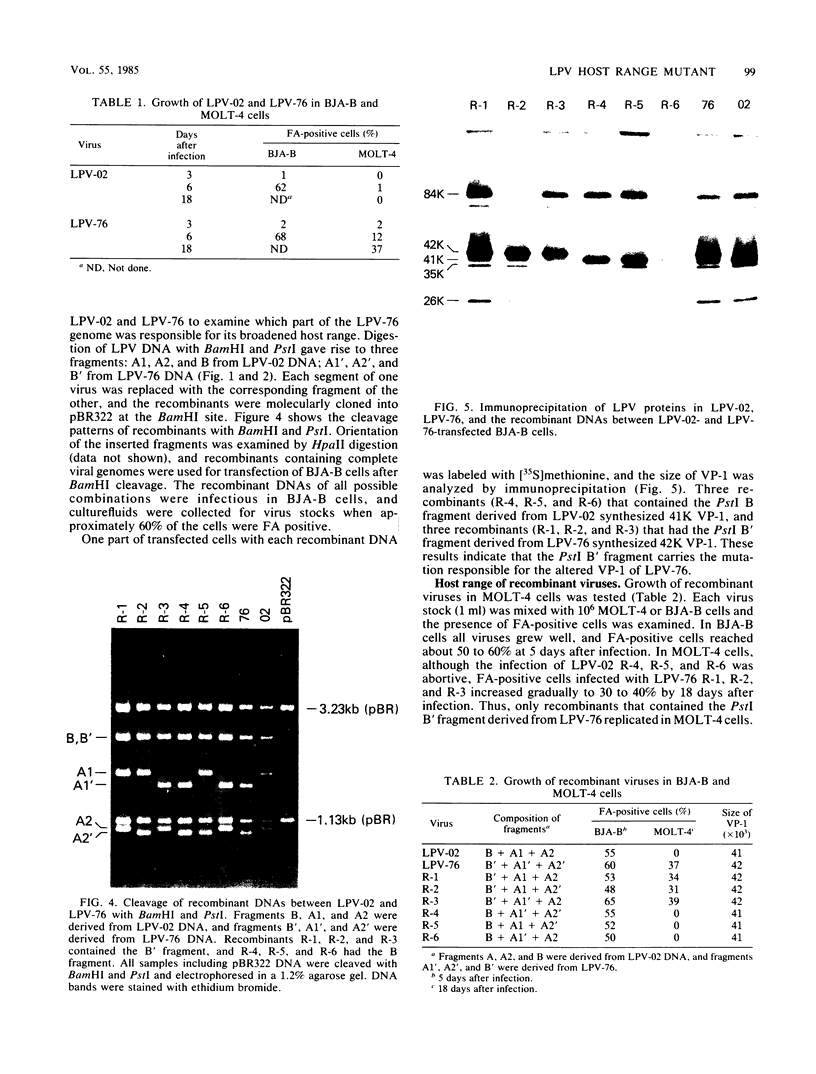
Monkey B-lymphotropic papovavirus (LPV) DNA present as free copies in LPV-transformed hamster embryo cells was molecularly cloned in Escherichia coli. Twenty-two of 24 cloned DNAs were 4.9 kilobases long and shorter than the wild-type LPV DNA (5.1 kilobases). The shorter DNA was nondefective and generated infectious virus (designated LPV-76) upon transfection of human B-lymphoblastoid BJA-B cells. LPV-76 DNA had a small deletion in the early region and a deletion and an insertion in the control region for transcription. LPV-76 VP-1 was apparently larger than that of the wild-type LPV. LPV-76 could grow in human T-lymphoblastoid MOLT-4 cells, whereas the wild-type LPV replicated only in B-lymphoblastoid cells. Characterization of constructed recombinant viruses between wild-type LPV and LPV-76 showed that the mutation responsible for the extended host range of LPV-76 was within the PstI B fragment, which includes the VP-1 coding region. These data strongly suggest that the mutation of VP-1 altered the host range of LPV.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade L., Müller-Lantzsch N., zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6(4):301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno A., Miyamura T., Yoshiike K. Monkey B-lymphotropic papovavirus DNA: nucleotide sequence of the region around the origin of replication. J Virol. 1984 May;50(2):451–456. doi: 10.1128/jvi.50.2.451-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kanda T., Yoshiike K., Takemoto K. K. Alignment of the genome of monkey B-lymphotropic papovavirus to the genomes of simian virus 40 and BK virus. J Virol. 1983 Apr;46(1):333–336. doi: 10.1128/jvi.46.1.333-336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J. The nature of the host range restriction of SV40 and polyoma viruses in embryonal carcinoma cells. Curr Top Microbiol Immunol. 1982;101:1–30. doi: 10.1007/978-3-642-68654-2_1. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai Srivastava B. I., Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1973 Apr 2;51(3):529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- Segawa K., Takemoto K. K. Identification of B-lymphotropic papovavirus-coded proteins. J Virol. 1983 Feb;45(2):872–875. doi: 10.1128/jvi.45.2.872-875.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Furuno A., Kato K., Yoshiike K. Biological and biochemical studies of African green monkey lymphotropic papovavirus. J Virol. 1982 May;42(2):502–509. doi: 10.1128/jvi.42.2.502-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Kanda T. Lymphotropic papovavirus transformation of hamster embryo cells. J Virol. 1984 Apr;50(1):100–105. doi: 10.1128/jvi.50.1.100-105.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Yoshiike K. Change of DNA near the origin of replication enhances the transforming capacity of human papovavirus BK. J Virol. 1982 Jun;42(3):978–985. doi: 10.1128/jvi.42.3.978-985.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike K., Miyamura T., Chan H. W., Takemoto K. K. Two defective DNAs of human polyomavirus JC adapted to growth in human embryonic kidney cells. J Virol. 1982 May;42(2):395–401. doi: 10.1128/jvi.42.2.395-401.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]