Abstract
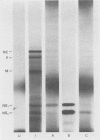
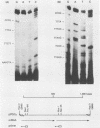
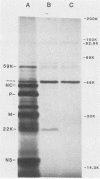
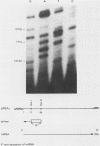
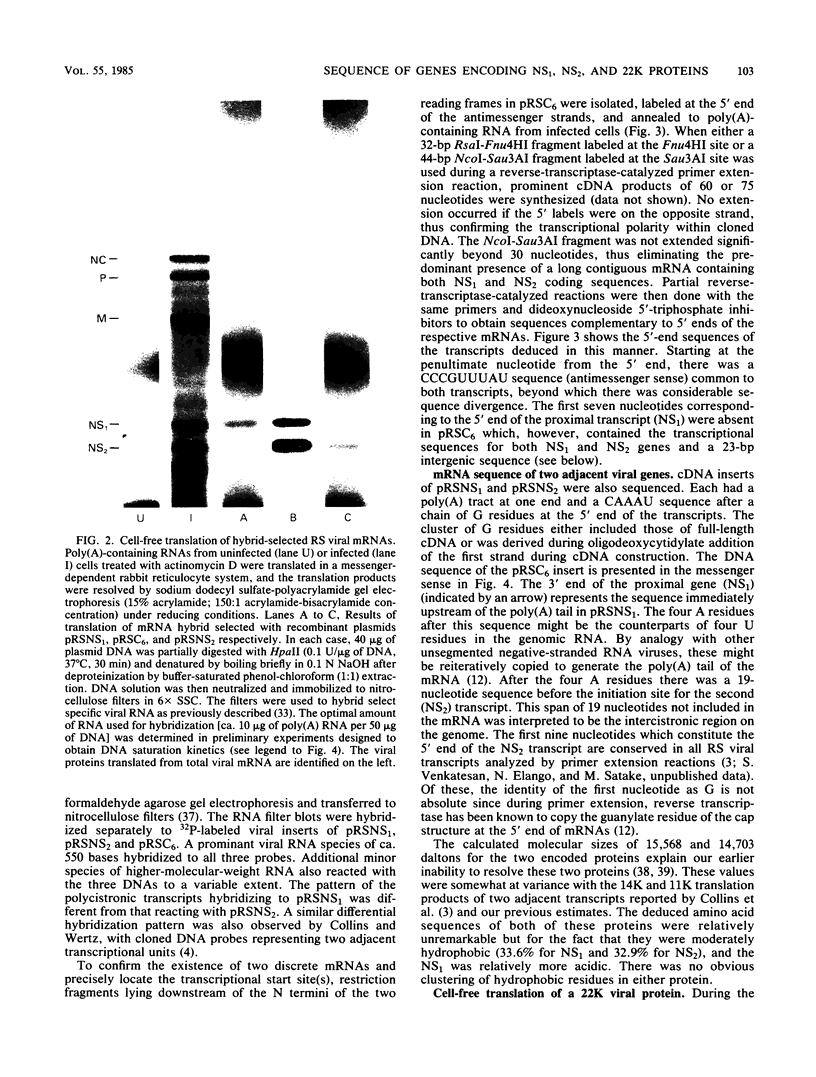
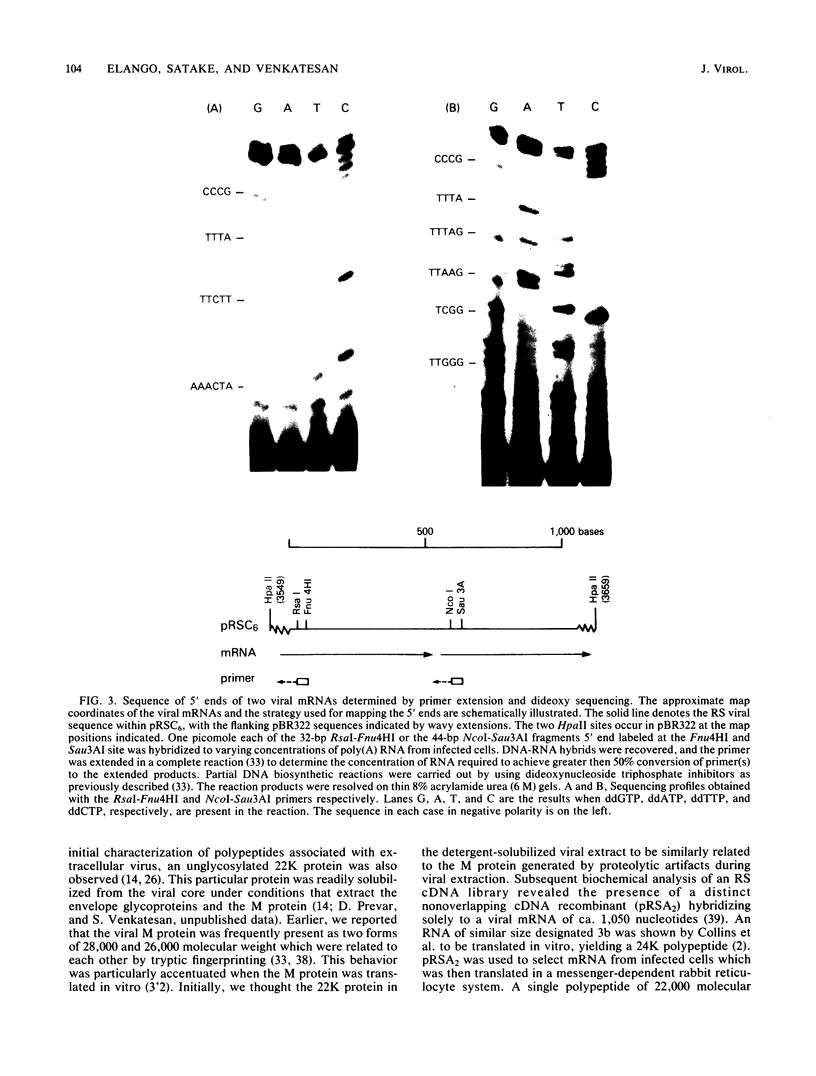
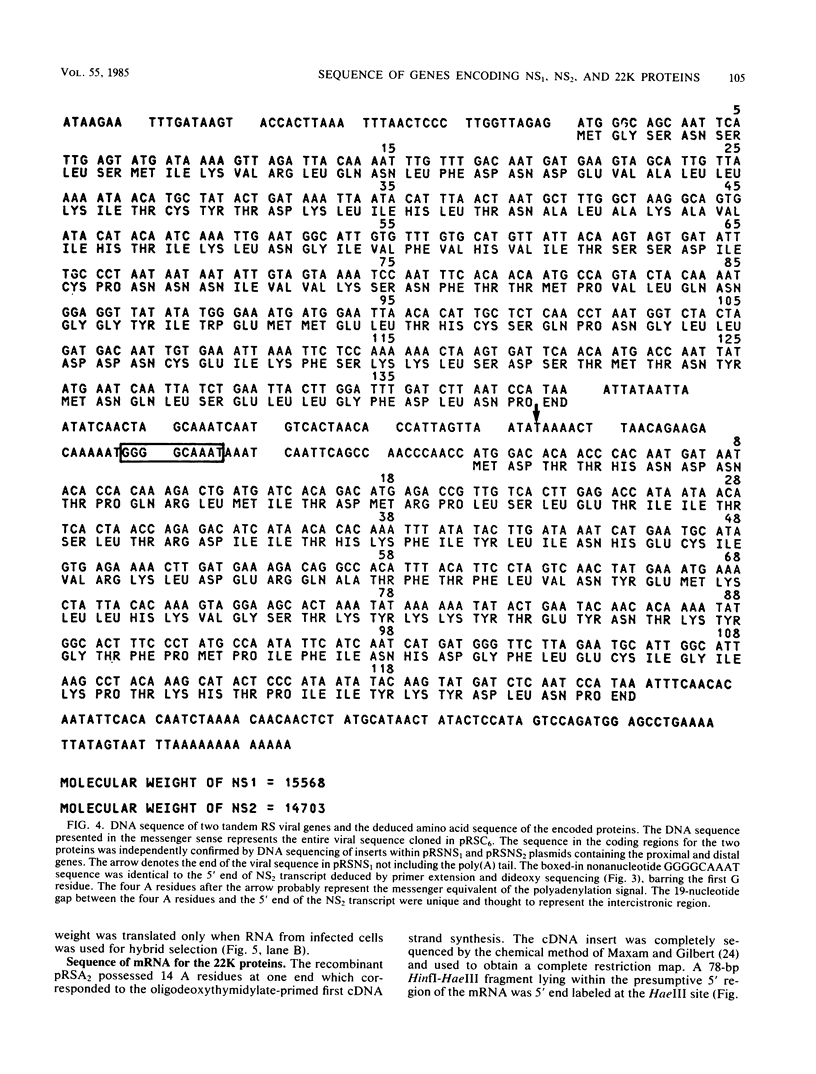
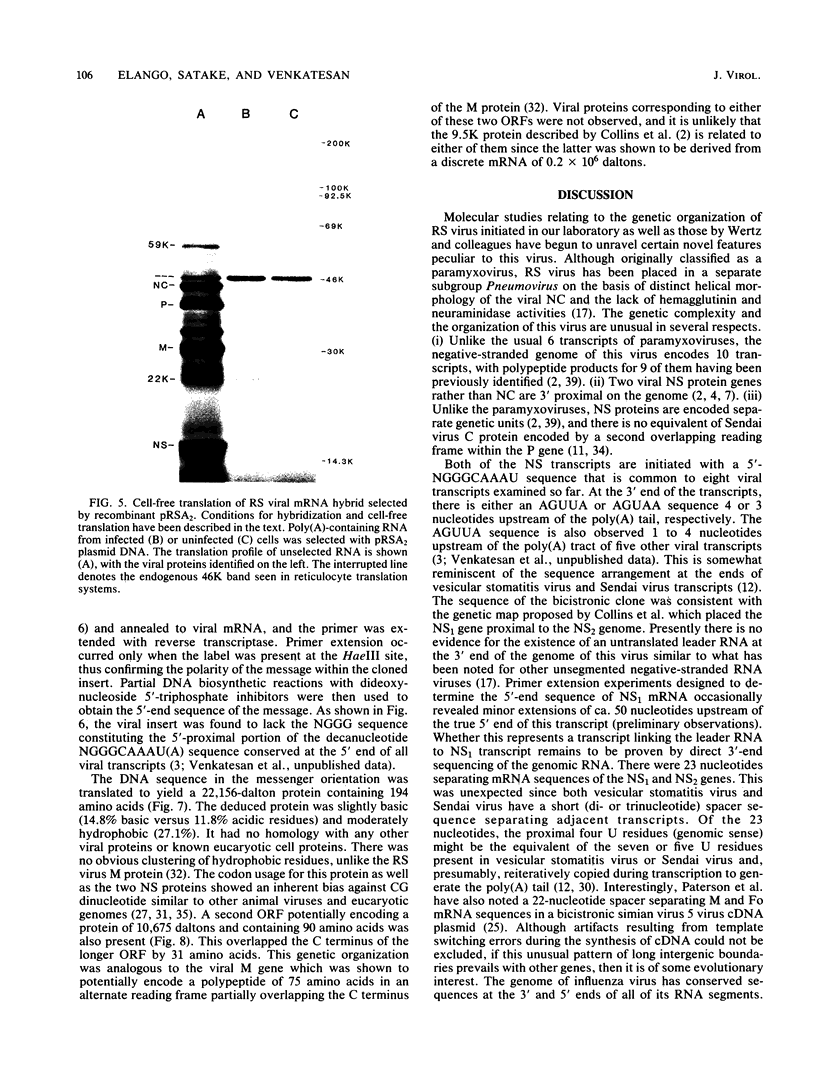
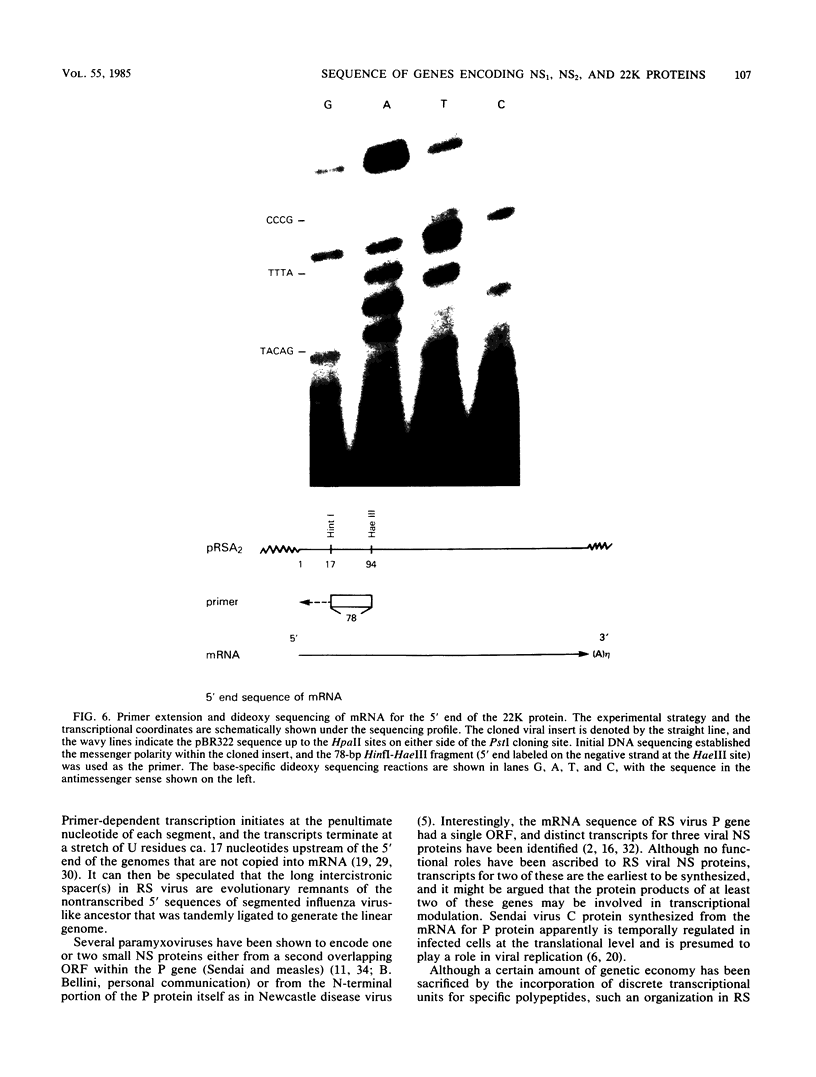
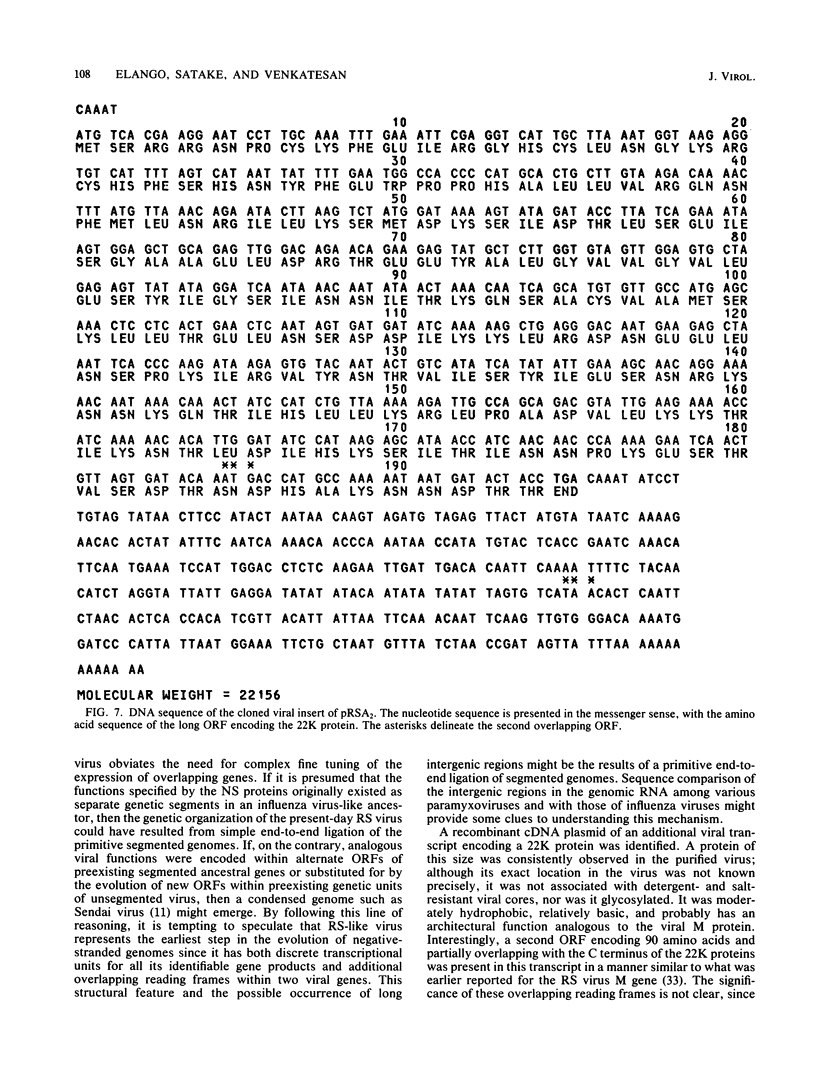
An mRNA sequence of two human respiratory syncytial viral nonstructural protein genes and of a gene for a 22,000-molecular-weight (22K) protein was obtained by cDNA cloning and DNA sequencing. Sequences corresponding to the 5' ends of the respective transcripts were deduced directly by primer extension and dideoxy nucleotide sequencing of the mRNAs. The availability of a bicistronic clone (pRSC6) confirmed the gene order for this portion of the genome. Contrary to other unsegmented negative-stranded RNA viruses, a 19-nucleotide intercistronic sequence was present between the NS1 and NS2 genes. The translation of cloned viral sequences in the bicistronic and monocistronic clones (pRSNS1 and pRSNS2) revealed two moderately hydrophobic proteins of 15,568 and 14,703 daltons. Their similarity in molecular size explained our earlier inability to resolve these proteins. A DNA sequence of an additional recombinant plasmid (pRSA2) revealed a long open reading frame encoding a 22,156-dalton protein containing 194 amino acids. It was relatively basic and moderately hydrophobic. A protein of this size was readily translated in vitro from a viral mRNA hybrid selected by this plasmid and corresponded to an unglycosylated 22K protein seen in purified extracellular virus but not associated with detergent- and salt-resistant cores. A second open reading frame of 90 amino acids partially overlapping with the C terminus of the 22K protein was also present within this sequence. This was reminiscent of the viral matrix protein gene which was previously shown by us to contain two overlapping reading frames. The finding of three additional viral transcripts encoding at least three identifiable proteins in human respiratory syncytial virus was a novel departure from the usual genetic organization of paramyxoviruses. The 5' ends of all three transcripts had a 5'NGGGCAAAU sequence that is common to all viral transcripts analyzed so far. Although there was no obvious homology immediately upstream of the polyadenylate tail, an AGUUA (AGUAA in the case of NS2) was present between 1 and 4 nucleotides upstream of the polyadenylate end of NS1 and 22K protein mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein J. M., Hruska J. F. Respiratory syncytial virus proteins: identification by immunoprecipitation. J Virol. 1981 Apr;38(1):278–285. doi: 10.1128/jvi.38.1.278-285.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Identification of a tenth mRNA of respiratory syncytial virus and assignment of polypeptides to the 10 viral genes. J Virol. 1984 Feb;49(2):572–578. doi: 10.1128/jvi.49.2.572-578.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Huang Y. T., Wertz G. W. Nucleotide sequence of the gene encoding the fusion (F) glycoprotein of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7683–7687. doi: 10.1073/pnas.81.24.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W., Ball L. A., Hightower L. E. Coding assignments of the five smaller mRNAs of Newcastle disease virus. J Virol. 1982 Sep;43(3):1024–1031. doi: 10.1128/jvi.43.3.1024-1031.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Wertz G. W. cDNA cloning and transcriptional mapping of nine polyadenylylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dethlefsen L., Kolakofsky D. In vitro synthesis of the nonstructural C protein of Sendai virus. J Virol. 1983 Apr;46(1):321–324. doi: 10.1128/jvi.46.1.321-324.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens L. E., Collins P. L., Wertz G. W. Transcriptional mapping of human respiratory syncytial virus. J Virol. 1984 Nov;52(2):364–369. doi: 10.1128/jvi.52.2.364-369.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J. Analysis of proteins synthesized in respiratory syncytial virus-infected cells. J Virol. 1982 May;42(2):372–378. doi: 10.1128/jvi.42.2.372-378.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Venkatesan S. Amino acid sequence of human respiratory syncytial virus nucleocapsid protein. Nucleic Acids Res. 1983 Sep 10;11(17):5941–5951. doi: 10.1093/nar/11.17.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie B. F., Gerin J. L. Immunochemical identification of viral and nonviral proteins of the respiratory syncytial virus virion. Infect Immun. 1982 Jul;37(1):243–249. doi: 10.1128/iai.37.1.243-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C., Blumberg B. M., Kolakofsky D. Sendai virus contains overlapping genes expressed from a single mRNA. Cell. 1983 Dec;35(3 Pt 2):829–836. doi: 10.1016/0092-8674(83)90115-0. [DOI] [PubMed] [Google Scholar]
- Gupta K. C., Kingsbury D. W. Complete sequences of the intergenic and mRNA start signals in the Sendai virus genome: homologies with the genome of vesicular stomatitis virus. Nucleic Acids Res. 1984 May 11;12(9):3829–3841. doi: 10.1093/nar/12.9.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. C., Schubert M., Keene J. D., Lazzarini R. A. Polycistronic vesicular stomatitis virus RNA transcripts. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4662–4665. doi: 10.1073/pnas.77.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. Respiratory syncytial virus mRNA coding assignments. J Virol. 1983 May;46(2):667–672. doi: 10.1128/jvi.46.2.667-672.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. T., Wertz G. W. The genome of respiratory syncytial virus is a negative-stranded RNA that codes for at least seven mRNA species. J Virol. 1982 Jul;43(1):150–157. doi: 10.1128/jvi.43.1.150-157.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The gene structure and replication of influenza virus. Annu Rev Biochem. 1983;52:467–506. doi: 10.1146/annurev.bi.52.070183.002343. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lambert D. M., Pons M. W. Respiratory syncytial virus glycoproteins. Virology. 1983 Oct 15;130(1):204–214. doi: 10.1016/0042-6822(83)90128-9. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Harris T. J., Lamb R. A. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6706–6710. doi: 10.1073/pnas.81.21.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeples M., Levine S. Respiratory syncytial virus polypeptides: their location in the virion. Virology. 1979 May;95(1):137–145. doi: 10.1016/0042-6822(79)90408-2. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S., Schubert M., Lazzarini R. A. Polyadenylation sites for influenza virus mRNA. J Virol. 1981 Apr;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Satake M., Elango N., Venkatesan S. Sequence analysis of the respiratory syncytial virus phosphoprotein gene. J Virol. 1984 Dec;52(3):991–994. doi: 10.1128/jvi.52.3.991-994.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Venkatesan S. Nucleotide sequence of the gene encoding respiratory syncytial virus matrix protein. J Virol. 1984 Apr;50(1):92–99. doi: 10.1128/jvi.50.1.92-99.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda T., Hidaka Y., Kanda T., Shibuta H., Nomoto A., Iwasaki K. Sequence of 3,687 nucleotides from the 3' end of Sendai virus genome RNA and the predicted amino acid sequences of viral NP, P and C proteins. Nucleic Acids Res. 1983 Nov 11;11(21):7317–7330. doi: 10.1093/nar/11.21.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Elango N., Chanock R. M. Construction and characterization of cDNA clones for four respiratory syncytial viral genes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1280–1284. doi: 10.1073/pnas.80.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]