Abstract
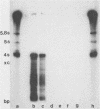
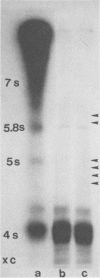
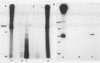
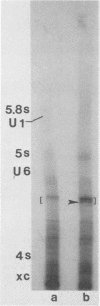
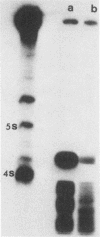
This study reports the partial characterization of nucleic acids present in gradient fractions enriched for large membrane vesicles from scrapie-infected and uninfected hamster brains. Labeling of phenol-extracted nucleic acids at the 3' or 5' ends revealed abundant amounts of low-molecular-weight RNA and little or no DNA. These nucleic acids survived nuclease treatment of membrane vesicles but were sensitive to RNase after phenol extraction. Analysis of 5'-end-labeled nucleic acids by one- and two-dimensional gel electrophoresis revealed an RNA of ca. 100 bases in preparations from scrapie-infected hamster brain that could not be detected in uninfected brain. The possibility that this apparently unique small RNA may result from tissue damage or abnormal RNA processing or may be a component of the infectious complex is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Young J. F., Palese P. Oligonucleotide mapping: evaluation of its sensitivity by computer-simulation. Nucleic Acids Res. 1982 Jan 11;10(1):237–246. doi: 10.1093/nar/10.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alper T., Cramp W. A., Haig D. A., Clarke M. C. Does the agent of scrapie replicate without nucleic acid? Nature. 1967 May 20;214(5090):764–766. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunel C., Widada J. S., Lelay M. N., Jeanteur P., Liautard J. P. Purification and characterization of a simple ribonucleoprotein particle containing small nucleoplasmic RNAs (snRNP) as a subset of RNP containing heterogenous nuclear RNA (hnRNP) from HeLa cells. Nucleic Acids Res. 1981 Feb 25;9(4):815–830. doi: 10.1093/nar/9.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees C., German T. L., Wade W. F., Marsh R. F. Characterization of lipids in membrane vesicles from scrapie-infected hamster brain. J Gen Virol. 1985 Apr;66(Pt 4):861–870. doi: 10.1099/0022-1317-66-4-861. [DOI] [PubMed] [Google Scholar]
- Dees C., German T. L., Wade W. F., Marsh R. F. Characterization of proteins in membrane vesicles from scrapie-infected hamster brain. J Gen Virol. 1985 Apr;66(Pt 4):851–859. doi: 10.1099/0022-1317-66-4-851. [DOI] [PubMed] [Google Scholar]
- Edmonds M., Vaughan M. H., Jr, Nakazato H. Polyadenylic acid sequences in the heterogeneous nuclear RNA and rapidly-labeled polyribosomal RNA of HeLa cells: possible evidence for a precursor relationship. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1336–1340. doi: 10.1073/pnas.68.6.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R. H., Marsh R. F. Comparison of scrapie and transmissible mink encephalopathy in hamsters. I. Biochemical studies of brain during development of disease. J Infect Dis. 1975 Feb;131(2):97–103. doi: 10.1093/infdis/131.2.97. [DOI] [PubMed] [Google Scholar]
- Latarjet R., Muel B., Haig D. A., Clarke M. C., Alper T. Inactivation of the scrapie agent by near monochromatic ultraviolet light. Nature. 1970 Sep 26;227(5265):1341–1343. doi: 10.1038/2271341a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Snurps and scyrps. Cell. 1981 Aug;25(2):298–300. doi: 10.1016/0092-8674(81)90047-7. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Castle B. E., Dees C., Wade W. F. Equilibrium density gradient centrifugation of the scrapie agent in Nycodenz. J Gen Virol. 1984 Nov;65(Pt 11):1963–1968. doi: 10.1099/0022-1317-65-11-1963. [DOI] [PubMed] [Google Scholar]
- Marsh R. F., Dees C., Castle B. E., Wade W. F., German T. L. Purification of the scrapie agent by density gradient centrifugation. J Gen Virol. 1984 Feb;65(Pt 2):415–421. doi: 10.1099/0022-1317-65-2-415. [DOI] [PubMed] [Google Scholar]
- Millson G. C., Hunter G. D., Kimberlin R. H. An experimental examination of the scrapie agent in cell membrane mixtures. II. The association of scrapie activity with membrane fractions. J Comp Pathol. 1971 Apr;81(2):255–265. doi: 10.1016/0021-9975(71)90100-9. [DOI] [PubMed] [Google Scholar]
- Millson G. C., Hunter G. D., Kimberlin R. H. The physico-chemical nature of the scrapie agent. Front Biol. 1976;44:243–266. [PubMed] [Google Scholar]
- Pattison I. H., Jebbett J. N. Clinical and histological observations on cuprizone toxicity and scrapie in mice. Res Vet Sci. 1971 Jul;12(4):378–380. [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. Analysis of the genome of an endogenous, ecotropic retrovirus of the AKR strain of mice: micromethod for detailed characterization of high-molecular-weight RNA. J Virol. 1980 Jan;33(1):349–365. doi: 10.1128/jvi.33.1.349-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., McKinley M. P., Bowman K. A., Bolton D. C., Bendheim P. E., Groth D. F., Glenner G. G. Scrapie prions aggregate to form amyloid-like birefringent rods. Cell. 1983 Dec;35(2 Pt 1):349–358. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- Sawyer R. C., Dahlberg J. E. Small RNAs of Rous sarcoma virus: characterization by two-dimensional polyacrylamide gel electrophoresis and fingerprint analysis. J Virol. 1973 Dec;12(6):1226–1237. doi: 10.1128/jvi.12.6.1226-1237.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Marsh R. F., Geelen J. L., Hanson R. P. Properties of the scrapie agent-endomembrane complex from hamster brain. J Virol. 1976 May;18(2):693–700. doi: 10.1128/jvi.18.2.693-700.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieve G. W. Two groups of small stable RNAs. Cell. 1981 Aug;25(2):296–297. doi: 10.1016/0092-8674(81)90046-5. [DOI] [PubMed] [Google Scholar]