Abstract
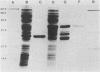
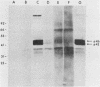
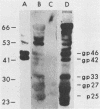
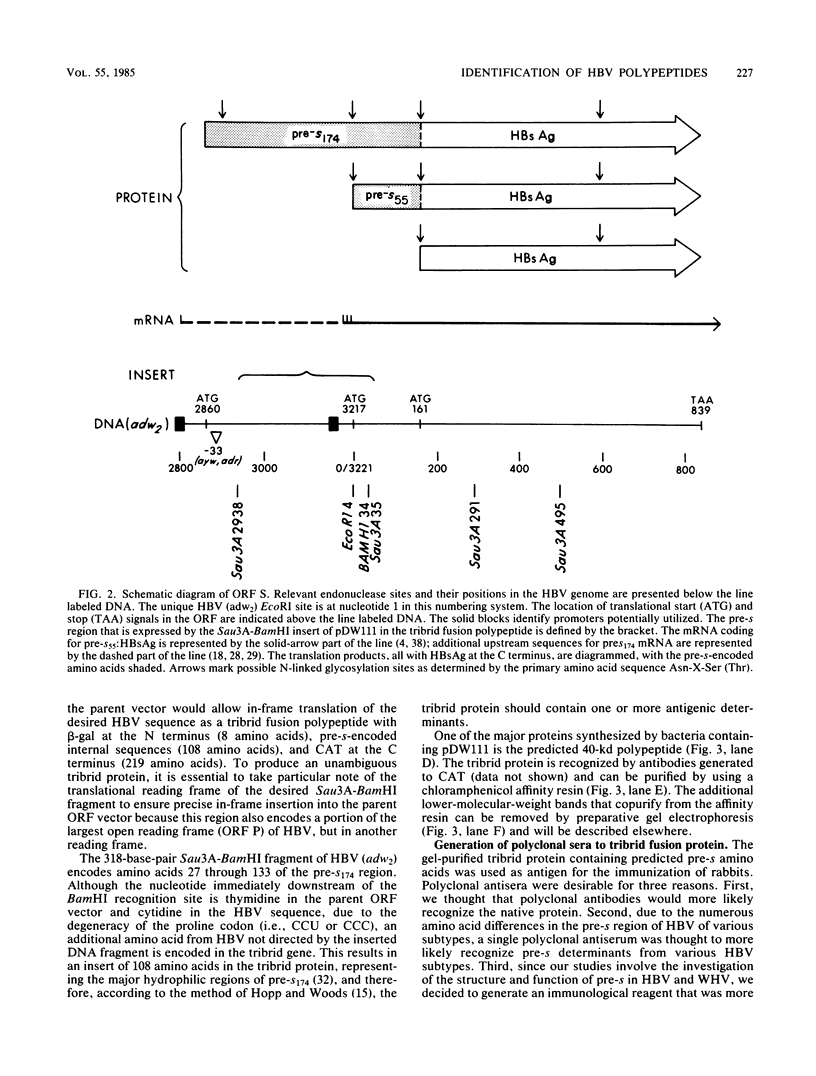
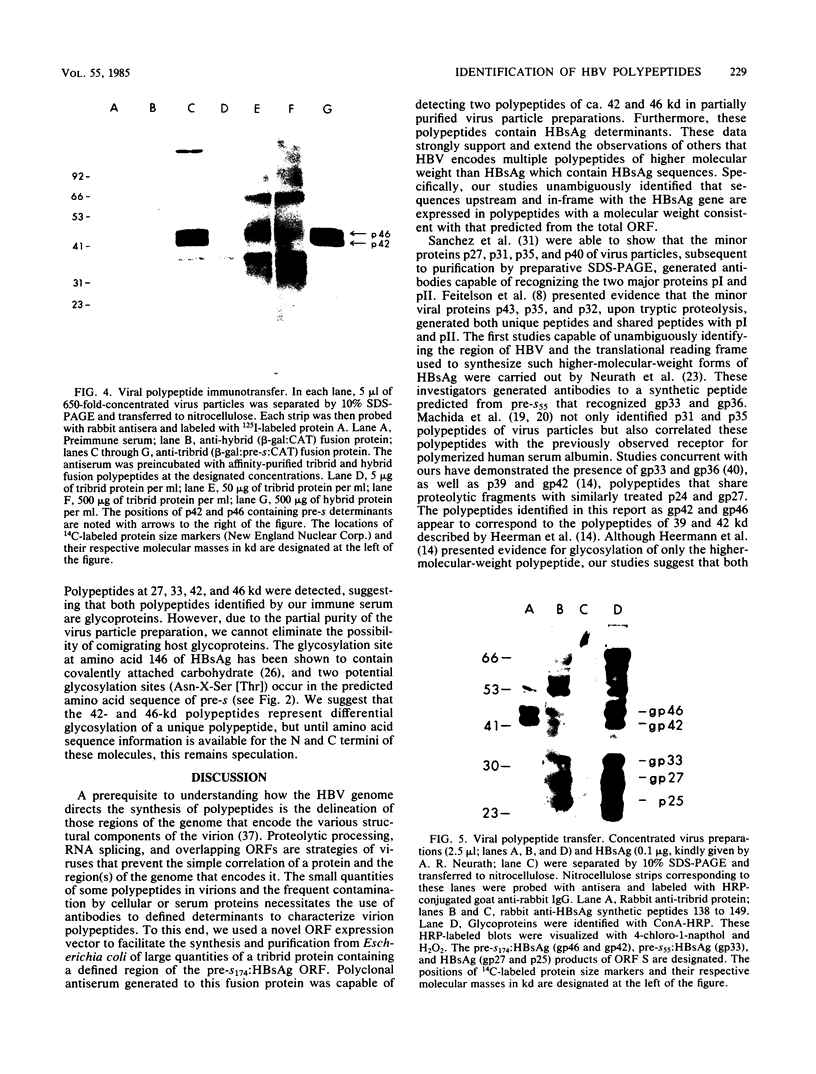
The open reading frame (ORF) that encodes the 226-amino-acid coat protein (hepatitis B virus surface antigen [HBsAg]) of hepatitis B virus has the potential to encode a 400-amino-acid polypeptide. The entire ORF would direct the synthesis of a polypeptide whose C-terminal amino acids represent HBsAg with an additional 174 amino acids at the N terminus (pre-s). Recently, virus particles have been shown to contain a polypeptide that corresponds to HBsAg with an additional 55 amino acids at the N terminus encoded by the DNA sequence immediately upstream of the HBsAg gene. A novel ORF expression vector containing the TAC promoter, the first eight codons of the gene for beta-galactosidase, and the entire coding sequence for chloramphenicol acetyltransferase was used in bacteria to express determinants of the 174 amino acids predicted from the pre-s portion of the ORF. The resulting tribrid protein containing 108 amino acids encoded by pre-s was expressed as one of the major proteins of bacteria harboring the recombinant plasmid. Single-step purification of the tribrid fusion protein was achieved by fractionation on a chloramphenicol affinity resin. Polyclonal antiserum generated to the fusion protein was capable of detecting 42- and 46-kilodalton polypeptides from virus particles; both polypeptides were also shown to contain HBsAg determinants. The ability of the polyclonal antiserum to identify polypeptides with these characteristics from virus particles presents compelling evidence that the DNA sequence of the entire ORF is expressed as a contiguous polypeptide containing HBsAg. The presence of multiple promoters and primary translation products from this single ORF argues that the function and potential interaction of the encoded polypeptides play a crucial role in the life cycle of the virus. Furthermore, the procedure and vector described in this report can be applied to other systems to facilitate the generation of antibodies to defined determinants and should allow the characterization of the epitope specificity of existing antibodies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti A., Pontisso P., Schiavon E., Realdi G. An antibody which precipitates Dane particles in acute hepatitis type B: relation to receptor sites which bind polymerized human serum albumin on virus particles. Hepatology. 1984 Mar-Apr;4(2):220–226. doi: 10.1002/hep.1840040209. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feitelson M. A., Marion P. L., Robinson W. S. The nature of polypeptides larger in size than the major surface antigen components of hepatitis b and like viruses in ground squirrels, woodchucks, and ducks. Virology. 1983 Oct 15;130(1):76–90. doi: 10.1016/0042-6822(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Rodriguez R. L., Doi R. H. Translational block to expression of the Escherichia coli Tn9-derived chloramphenicol-resistance gene in Bacillus subtilis. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5886–5890. doi: 10.1073/pnas.79.19.5886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Heermann K. H., Goldmann U., Schwartz W., Seyffarth T., Baumgarten H., Gerlich W. H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984 Nov;52(2):396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T. P., Woods K. R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai M., Yanase Y., Nojiri T., Miyakawa Y., Mayumi M. A receptor for polymerized human and chimpanzee albumins on hepatitis B virus particles co-occurring with HBeAg. Gastroenterology. 1979 Feb;76(2):242–247. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laub O., Rall L. B., Truett M., Shaul Y., Standring D. N., Valenzuela P., Rutter W. J. Synthesis of hepatitis B surface antigen in mammalian cells: expression of the entire gene and the coding region. J Virol. 1983 Oct;48(1):271–280. doi: 10.1128/jvi.48.1.271-280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Baba K., Ito Y., Miyamoto H., Funatsu G., Oda K., Usuda S., Togami S. A polypeptide containing 55 amino acid residues coded by the pre-S region of hepatitis B virus deoxyribonucleic acid bears the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1984 May;86(5 Pt 1):910–918. [PubMed] [Google Scholar]
- Machida A., Kishimoto S., Ohnuma H., Miyamoto H., Baba K., Oda K., Nakamura T., Miyakawa Y., Mayumi M. A hepatitis B surface antigen polypeptide (P31) with the receptor for polymerized human as well as chimpanzee albumins. Gastroenterology. 1983 Aug;85(2):268–274. [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath A. R., Kent S. B., Strick N. Location and chemical synthesis of a pre-S gene coded immunodominant epitope of hepatitis B virus. Science. 1984 Apr 27;224(4647):392–395. doi: 10.1126/science.6200931. [DOI] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasek M., Goto T., Gilbert W., Zink B., Schaller H., MacKay P., Leadbetter G., Murray K. Hepatitis B virus genes and their expression in E. coli. Nature. 1979 Dec 6;282(5739):575–579. doi: 10.1038/282575a0. [DOI] [PubMed] [Google Scholar]
- Peterson D. L. Isolation and characterization of the major protein and glycoprotein of hepatitis B surface antigen. J Biol Chem. 1981 Jul 10;256(13):6975–6983. [PubMed] [Google Scholar]
- Peterson D. L., Roberts I. M., Vyas G. N. Partial amino acid sequence of two major component polypeptides of hepatitis B surface antigen. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1530–1534. doi: 10.1073/pnas.74.4.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel C., Louise A., Gervais M., Chenciner N., Dubois M. F., Tiollais P. Transcription of the hepatitis B surface antigen gene in mouse cells transformed with cloned viral DNA. J Virol. 1982 Apr;42(1):100–105. doi: 10.1128/jvi.42.1.100-105.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Sanchez Y., Ionescu-Matiu I., Dreesman G. R., Hollinger F. B., Melnick J. L. Evidence for the presence of repeating antigenic determinants in the major polypeptides derived from hepatitis B surface antigen. Virology. 1981 Oct 15;114(1):71–80. doi: 10.1016/0042-6822(81)90253-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer E., Sninsky J. J. Predicted secondary structure similarity in the absence of primary amino acid sequence homology: hepatitis B virus open reading frames. Proc Natl Acad Sci U S A. 1984 May;81(9):2902–2906. doi: 10.1073/pnas.81.9.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottel J. L., Sninsky J. J., Cohen S. N. Effects of alterations in the translation control region on bacterial gene expression: use of cat gene constructs transcribed from the lac promoter as a model system. Gene. 1984 May;28(2):177–193. doi: 10.1016/0378-1119(84)90255-5. [DOI] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sninsky J. J., Siddiqui A., Robinson W. S., Cohen S. N. Cloning and endonuclease mapping of the hepatitis B viral genome. Nature. 1979 May 24;279(5711):346–348. doi: 10.1038/279346a0. [DOI] [PubMed] [Google Scholar]
- Standring D. N., Rutter W. J., Varmus H. E., Ganem D. Transcription of the hepatitis B surface antigen gene in cultured murine cells initiates within the presurface region. J Virol. 1984 May;50(2):563–571. doi: 10.1128/jvi.50.2.563-571.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Structural relationships between minor and major proteins of hepatitis B surface antigen. J Virol. 1983 May;46(2):626–628. doi: 10.1128/jvi.46.2.626-628.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbe W., Gerlich W. H. Variable protein composition of hepatitis B surface antigen from different donors. Virology. 1982 Dec;123(2):436–442. doi: 10.1016/0042-6822(82)90275-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vyas G. N., Rao K. R., Ibrahim A. B. Australia antigen (hepatitis B antigen): a conformational antigen dependent on disulfide bonds. Science. 1972 Dec 22;178(4067):1300–1301. doi: 10.1126/science.178.4067.1300. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]