Abstract
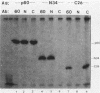
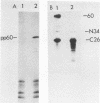
Antisera to the Rous sarcoma virus transforming protein pp60src were produced in rabbits immunized with p60src purified from recombinant Escherichia coli. Immunization with isolated V8 proteolytic fragments of p60src resulted in antisera specific for the amino- and carboxy-terminal domains. The reactions of these antisera with pp60v-src and the normal cell protein p60c-src were characterized.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Antigenic and structural studies on the transforming proteins of Rous sarcoma virus and Yamaguchi 73 avian sarcoma virus. Virology. 1983 Oct 15;130(1):221–226. doi: 10.1016/0042-6822(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Development of anti-pp60src serum with antigen produced in Escherichia coli. J Virol. 1983 Jan;45(1):462–465. doi: 10.1128/jvi.45.1.462-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature. 1981 Dec 24;294(5843):771–773. doi: 10.1038/294771a0. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Parsons J. T., Erikson R. L. Construction of plasmids for expression of Rous sarcoma virus transforming protein, p60src, in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2152–2156. doi: 10.1073/pnas.79.7.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Courtneidge S. A., Bishop J. M. Structural and functional domains of the Rous sarcoma virus transforming protein (pp60src). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1624–1628. doi: 10.1073/pnas.78.3.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Sefton B. M., Hunter T., Walter G., Singer S. J. Immunofluorescent localization of the transforming protein of Rous sarcoma virus with antibodies against a synthetic src peptide. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5322–5326. doi: 10.1073/pnas.79.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Isolation and partial characterization of a monoclonal antibody to the Rous sarcoma virus transforming protein pp60src. J Virol. 1983 Mar;45(3):1190–1194. doi: 10.1128/jvi.45.3.1190-1194.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984 Aug;51(2):272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Characterization of pp60src phosphorylation in vitro in Rous sarcoma virus-transformed cell membranes. Mol Cell Biol. 1985 May;5(5):916–922. doi: 10.1128/mcb.5.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985 Feb;100(2):409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Walter G. Antiserum specific for the carboxy terminus of the transforming protein of Rous sarcoma virus. J Virol. 1982 Nov;44(2):467–474. doi: 10.1128/jvi.44.2.467-474.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel M. B., Sinha Y. N., VanderLaan W. P. Production of antibodies by inoculation into lymph nodes. Methods Enzymol. 1983;93:3–12. doi: 10.1016/s0076-6879(83)93031-8. [DOI] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T., Bauer H., Birr C., Pipkorn R. Antibodies against synthetic peptides as a tool for functional analysis of the transforming protein pp60src. Cell. 1983 Sep;34(2):587–596. doi: 10.1016/0092-8674(83)90391-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. W., Goldberg A. R. Synthetic peptide fragment of src gene product inhibits the src protein kinase and crossreacts immunologically with avian onc kinases and cellular phosphoproteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7412–7416. doi: 10.1073/pnas.78.12.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]