Abstract
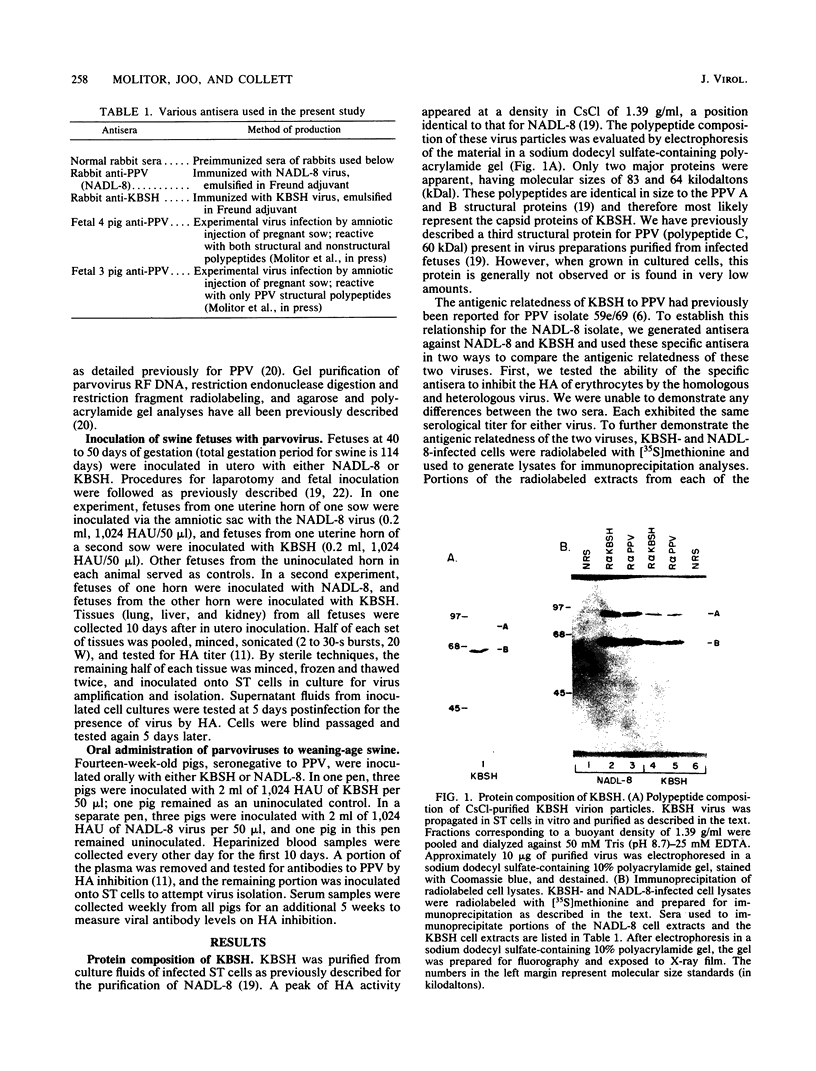
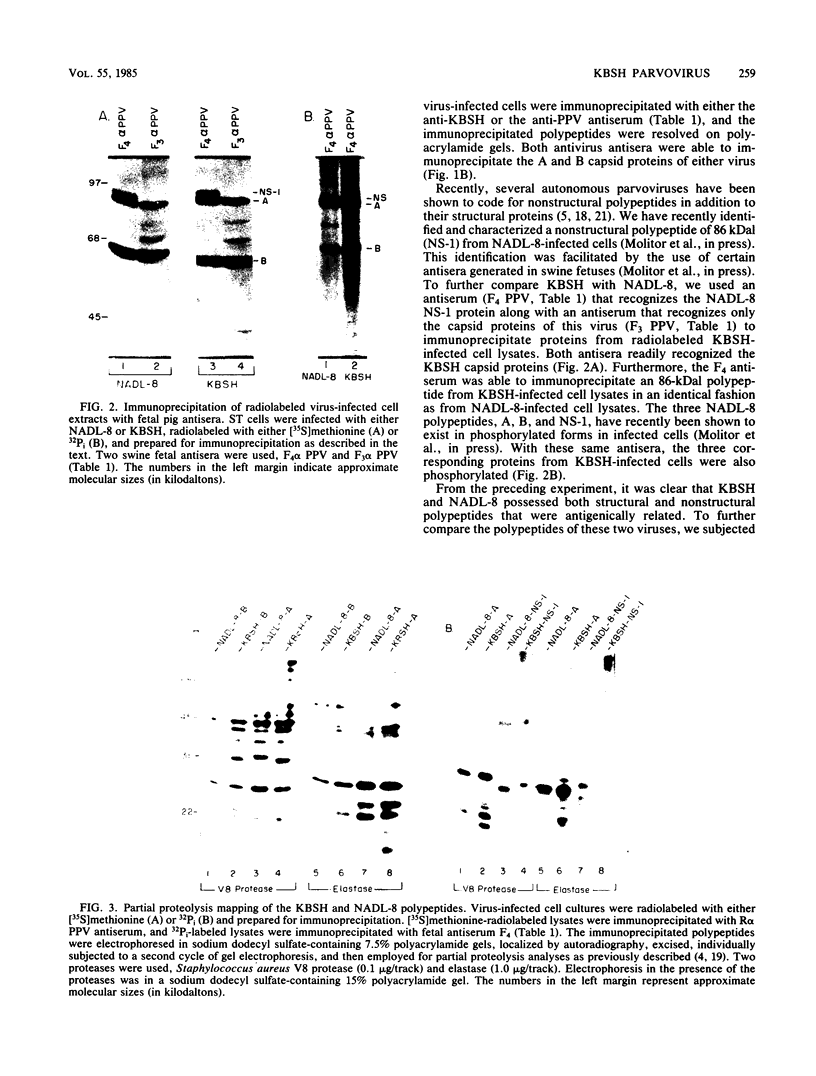
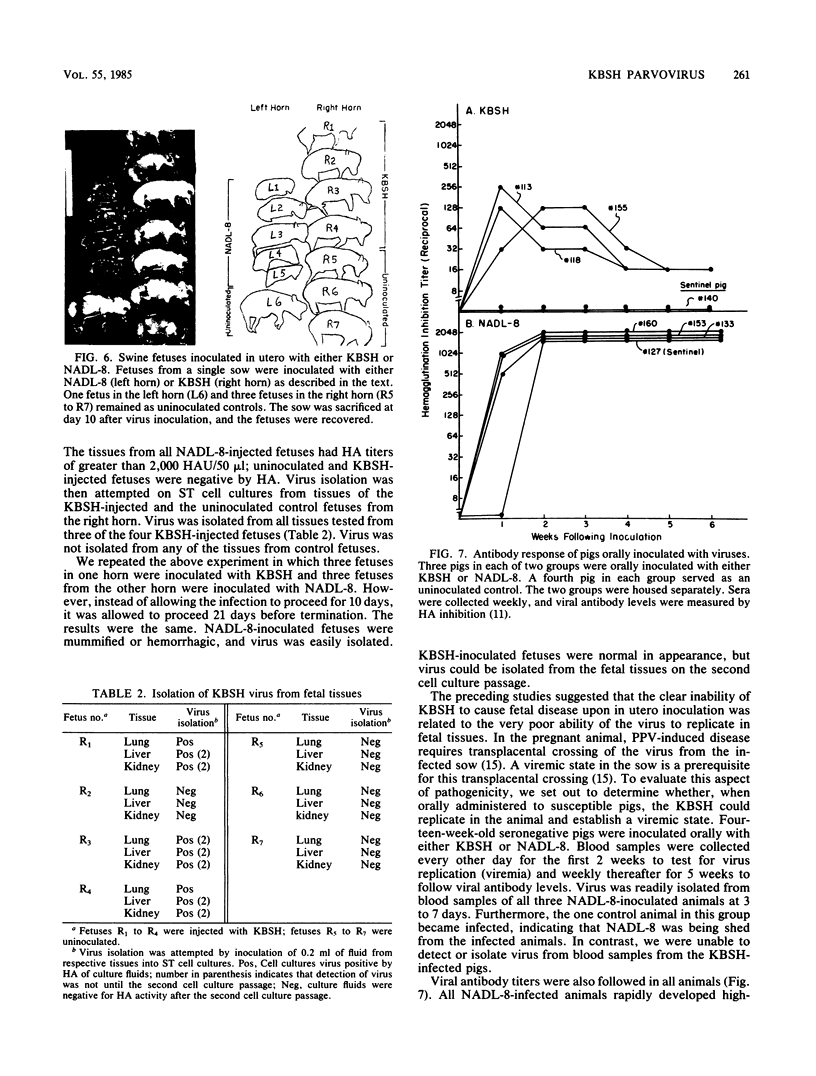
We compared the molecular, antigenic, and pathogenic properties of KBSH parvovirus to those of porcine parvovirus (PPV) isolate NADL-8. KBSH, propagated in swine testes cells in culture, possessed two major capsid polypeptides of 83 and 64 kilodaltons that were similar in size to those of PPV. KBSH-infected cells also contained an 86-kilodalton nonstructural polypeptide that was identical in size to the PPV nonstructural polypeptide (NS-1). The KBSH polypeptides were structurally similar but not identical to the corresponding PPV polypeptides, as revealed by partial proteolysis mapping. Viral replicative-form DNA from KBSH-infected cells was similar in size to PPV replicative-form DNA and exhibited similar but not identical restriction endonuclease cleavage patterns to that of PPV replicative-form DNA. Antigenically, the two viruses were also very closely related. By using heterologous and homologous antisera, the two viruses were indistinguishable in hemagglutination inhibition and immunoprecipitation assays. However, pathogenically these viruses were dramatically different. NADL-8 caused fetal death when injected into swine fetuses in utero and viremia and high persisting antibody titers when administered orally to weaning-age swine. KBSH-inoculated fetuses were normal in appearance, and pigs orally exposed to KBSH failed to establish viremia and demonstrated only transient antibody titers. Thus, KBSH appears to be a PPV that is very closely related to a highly pathogenic PPV isolate, yet is itself nonpathogenic in swine. This reduced pathogenic potential of KBSH may be attributable to its poor ability to replicate in swine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cartwright S. F., Lucas M., Huck R. A. A small haemagglutinating porcine DNA virus. I. Isolation and properties. J Comp Pathol. 1969 Jul;79(3):371–377. doi: 10.1016/0021-9975(69)90053-x. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cotmore S. F., Sturzenbecker L. J., Tattersall P. The autonomous parvovirus MVM encodes two nonstructural proteins in addition to its capsid polypeptides. Virology. 1983 Sep;129(2):333–343. doi: 10.1016/0042-6822(83)90172-1. [DOI] [PubMed] [Google Scholar]
- Hallauer C., Kronauer G., Siegl G. Parvoiruses as contaminants of permanent human cell lines. I. Virus isolation from 1960-1970. Arch Gesamte Virusforsch. 1971;35(1):80–90. doi: 10.1007/BF01249755. [DOI] [PubMed] [Google Scholar]
- Hallauer C., Siegl G., Kronauer G. Parvoviruses as contaminants of permanent human cell lines. 3. Biological properties of the isolated viruses. Arch Gesamte Virusforsch. 1972;38(4):366–382. doi: 10.1007/BF01262827. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. H., Collings D. F. Experimental infection of piglets and pregnant gilts with a parvovirus. Vet Rec. 1969 Oct 18;85(16):446–447. doi: 10.1136/vr.85.16.446. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Donaldson-Wood C. R., Johnson R. H. A standardised haemagglutination inhibition test for porcine parvovirus antibody. Aust Vet J. 1976 Sep;52(9):422–424. doi: 10.1111/j.1751-0813.1976.tb09517.x. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Donaldson-Wood C. R., Johnson R. H. Observations on the pathogenesis of porcine parvovirus infection. Arch Virol. 1976;51(1-2):123–129. doi: 10.1007/BF01317841. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Johnson R. H. Serological responses in pigs vaccinated with inactivated porcine parvovirus. Aust Vet J. 1977 Nov;53(11):550–552. doi: 10.1111/j.1751-0813.1977.tb07945.x. [DOI] [PubMed] [Google Scholar]
- Joo H. S., Molitor T. W., Leman A. D. Antibody responses of guinea-pigs, rabbits and pigs to inactivated porcine parvovirus vaccines. Vet Microbiol. 1984 Feb;9(1):27–33. doi: 10.1016/0378-1135(84)90076-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mengeling W. L., Cutlip R. C. Pathogenesis of in utero infection: experimental infection of five-week-old porcine fetuses with porcine parvovirus. Am J Vet Res. 1975 Aug;36(08):1173–1177. [PubMed] [Google Scholar]
- Mengeling W. L., Cutlip R. C. Reproductive disease experimentally induced by exposing pregnant gilts to porcine parvovirus. Am J Vet Res. 1976 Dec;37(12):1393–1400. [PubMed] [Google Scholar]
- Mitra R., Wali T., Valdez V., Fabisch P., Salzman L. A. Transcription and translation in the autonomous parvovirus KRV. Virology. 1983 Mar;125(2):349–360. doi: 10.1016/0042-6822(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus DNA: characterization of the genomic and replicative form DNA of two virus isolates. Virology. 1984 Sep;137(2):241–254. doi: 10.1016/0042-6822(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Molitor T. W., Joo H. S., Collett M. S. Porcine parvovirus: virus purification and structural and antigenic properties of virion polypeptides. J Virol. 1983 Feb;45(2):842–854. doi: 10.1128/jvi.45.2.842-854.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Identification of multiple forms of the noncapsid parvovirus protein NCVP1 in H-1 parvovirus-infected cells. J Virol. 1984 Oct;52(1):82–87. doi: 10.1128/jvi.52.1.82-87.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman D. R., Bohl E. H., Ferguson L. C. Porcine parvovirus: natural and experimental infections of the porcine fetus and prevalence in mature swine. Infect Immun. 1974 Oct;10(4):718–723. doi: 10.1128/iai.10.4.718-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G. Parvoviruses as contaminants of permanent human cell lines. V. The nucleic acid of KBSH-virus. Arch Gesamte Virusforsch. 1972;37(2):267–274. doi: 10.1007/BF01268010. [DOI] [PubMed] [Google Scholar]
- Sørensen K. J., Askaa J. Vaccination against porcine parvovirus infection. Acta Vet Scand. 1981;22(2):171–179. doi: 10.1186/BF03547506. [DOI] [PMC free article] [PubMed] [Google Scholar]