Abstract
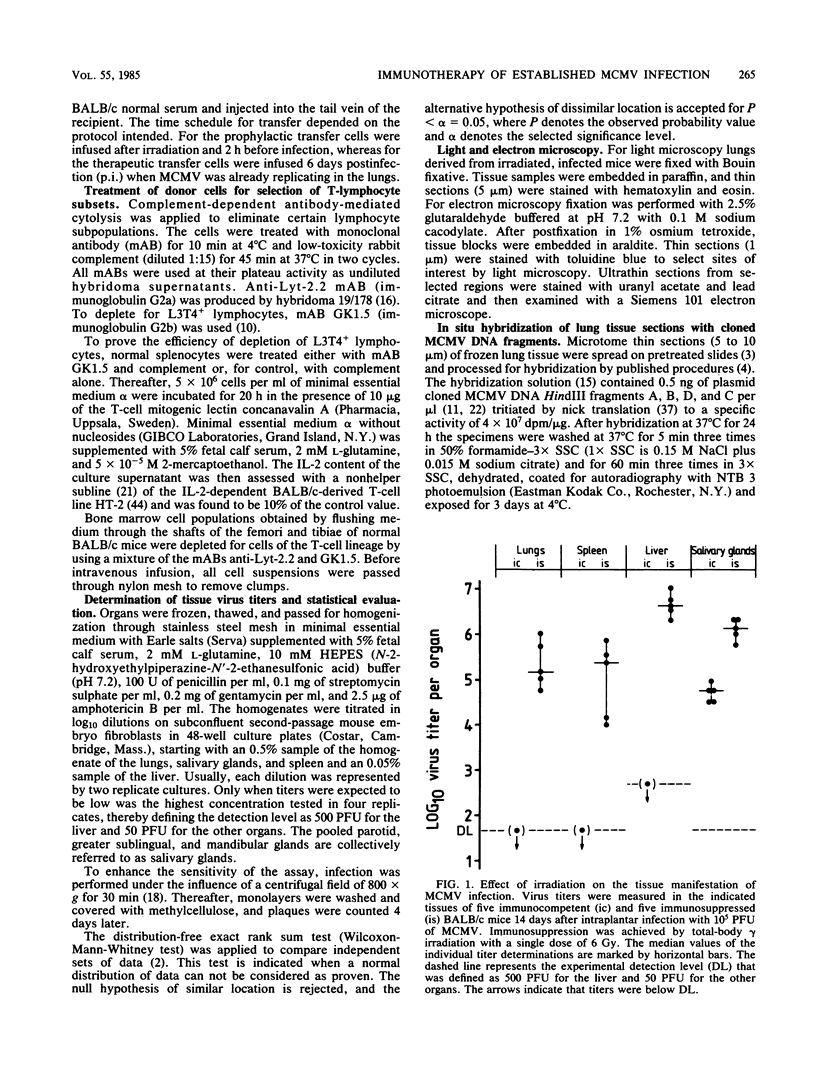
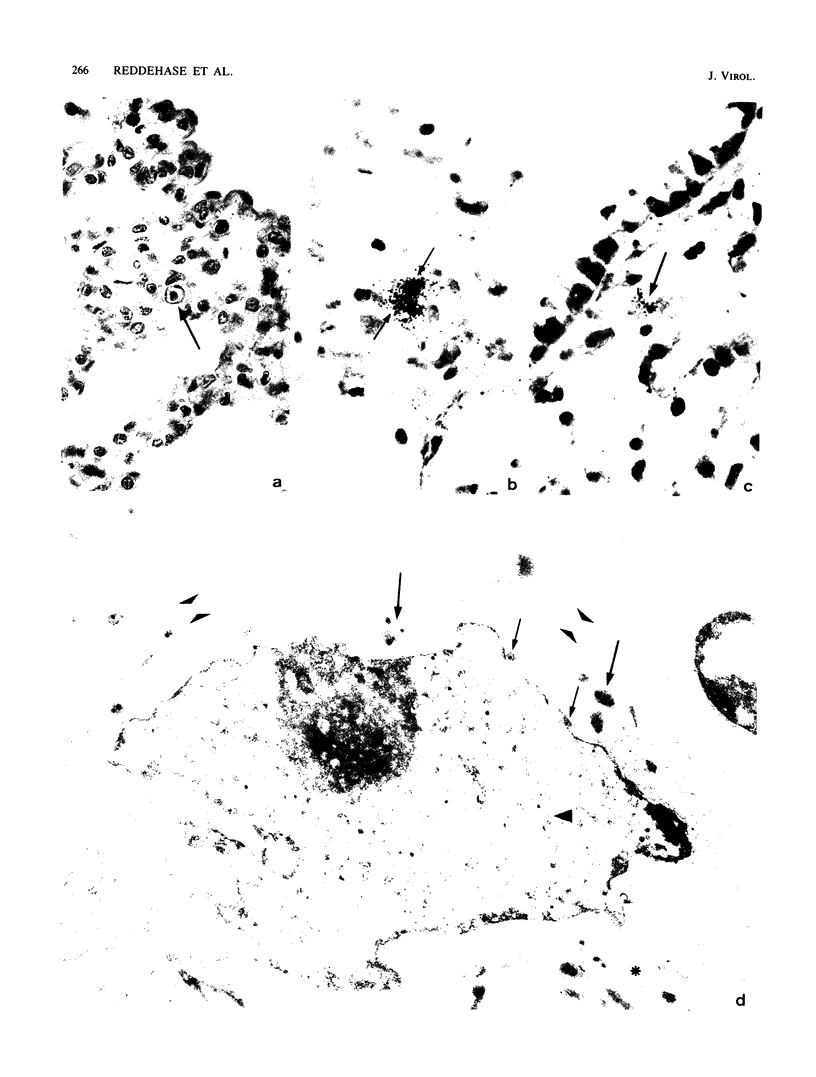
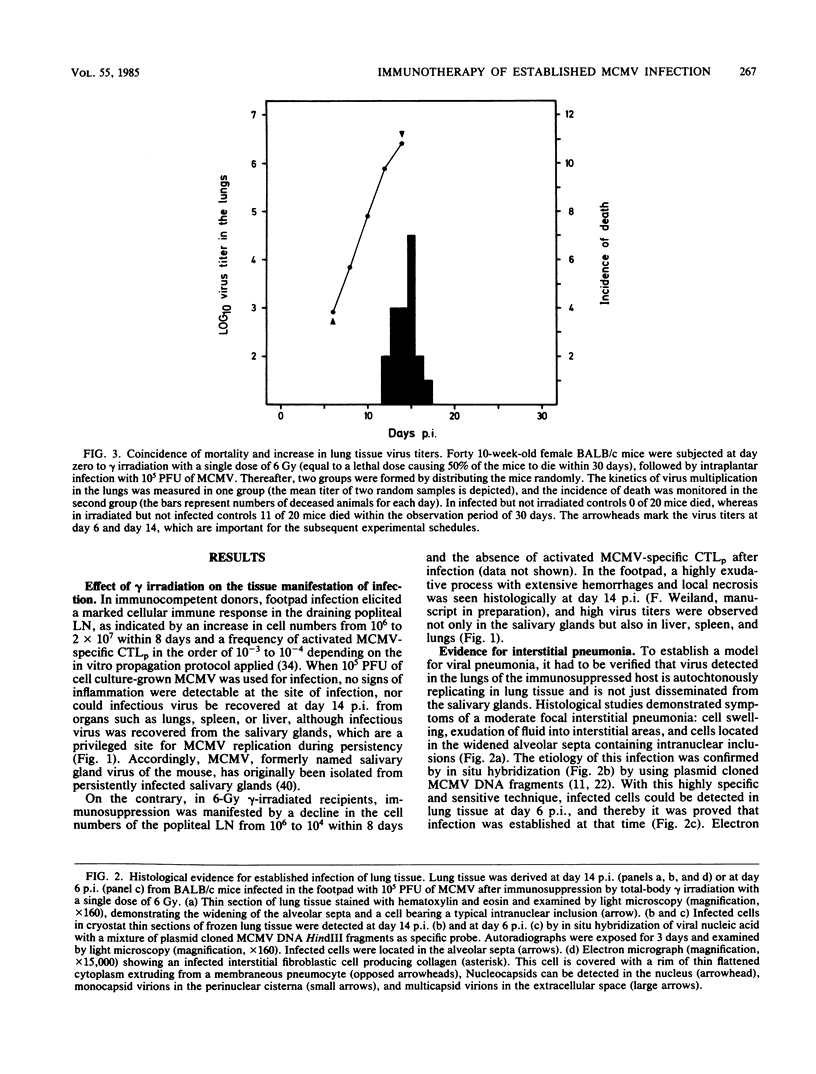
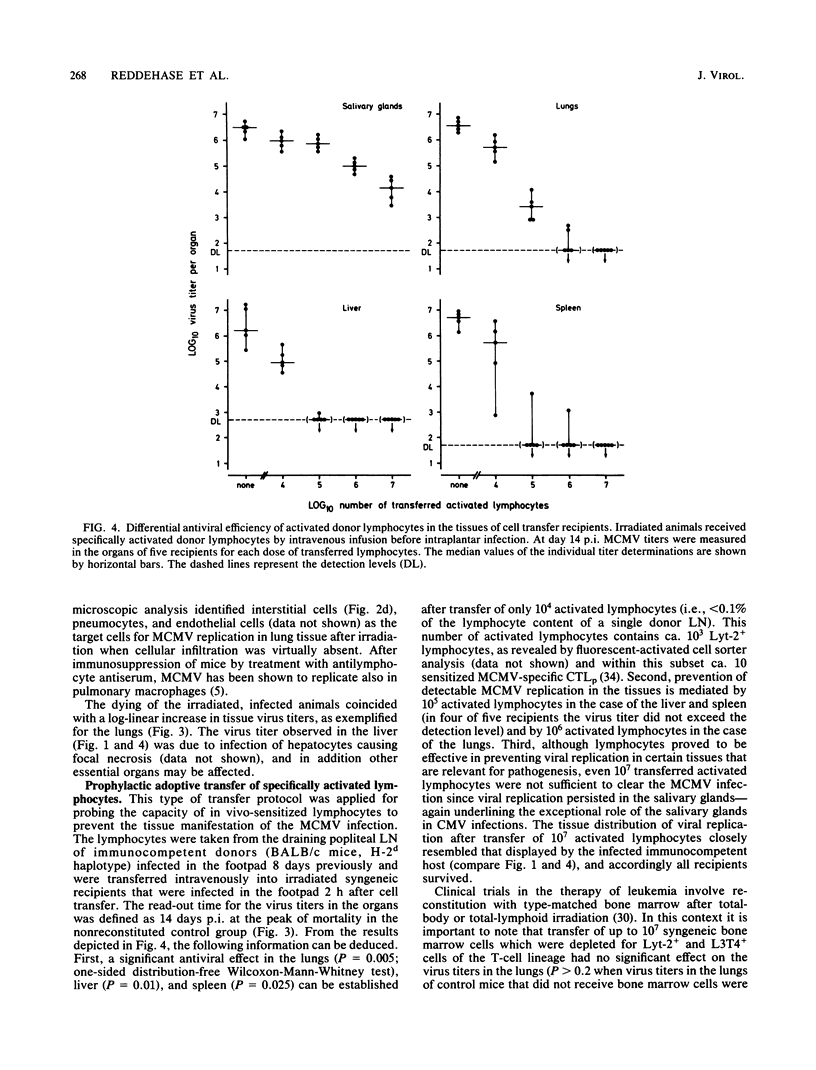
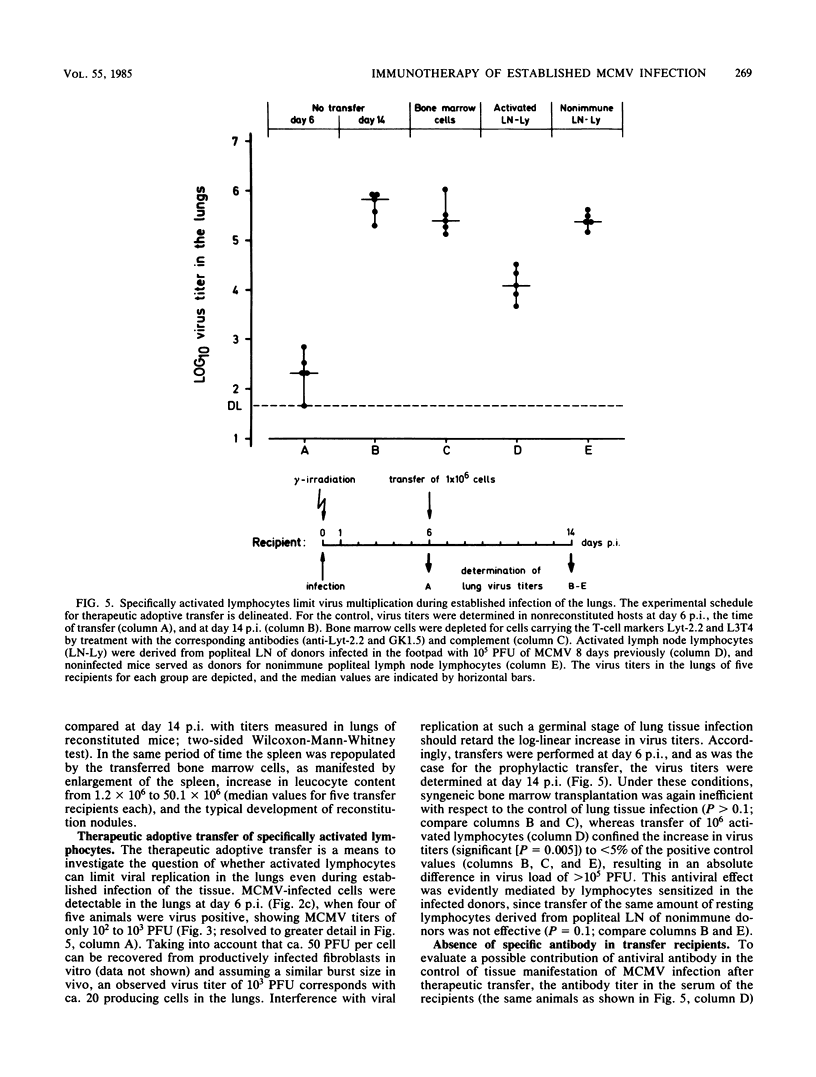
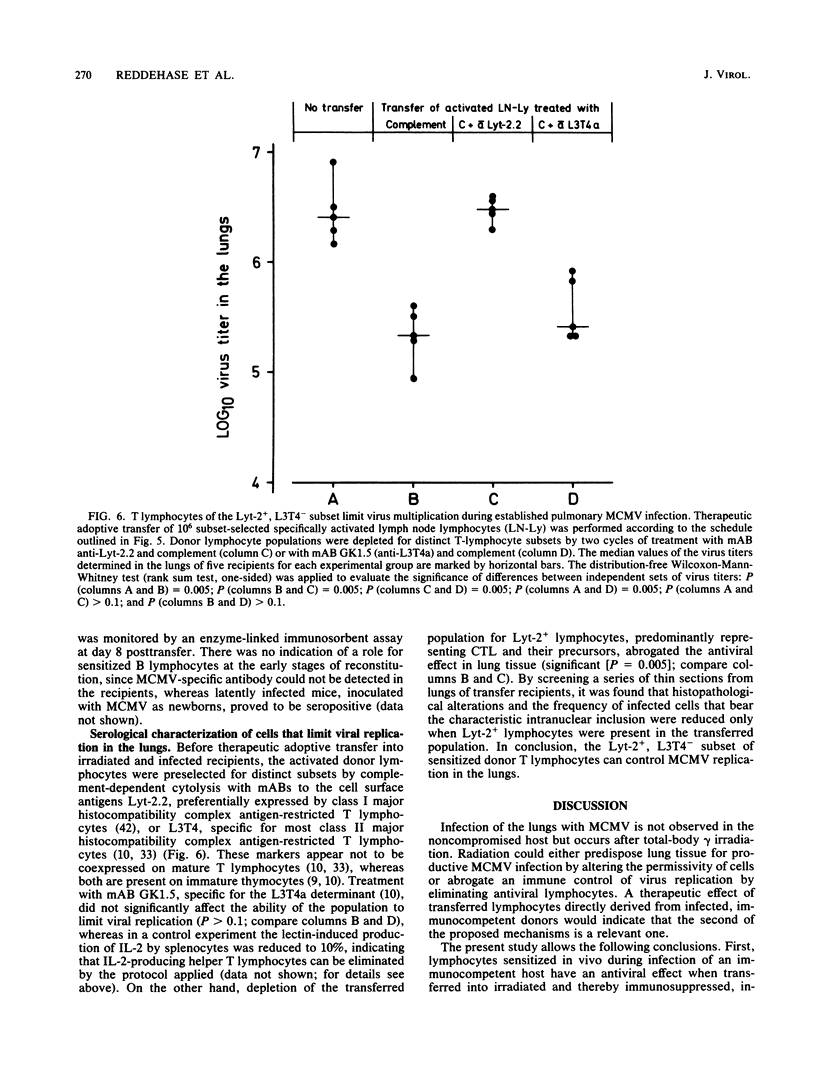
Interstitial pneumonia associated with viral replication in lung tissue was observed after cytomegalovirus infection of total-body gamma-irradiated mice, whereas in noncompromised hosts the lungs were not affected and virus multiplication was restricted to the salivary glands. The radiation damage could either predispose normally nonpermissive cell types for productive infection or abrogate an immune control of the tissue manifestation of infection by elimination of lymphocytes. Adoptive transfer of lymphoid cells into irradiated, infected recipients supported the second alternative. Even when infection was established in the lungs, as manifested by the presence of infected lung tissue cells in the alveolar septa, an antiviral effect could be assigned to the Lyt-2+, L3T4- subset of T lymphocytes specifically sensitized in the immunocompetent donor. These cells did not require in vitro propagation to perform effector cell functions in vivo and were operative under physiological conditions in comparatively low numbers. Hence, there is reason to assume that T lymphocytes are responsible for the tissue distribution of cytomegalovirus replication during infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971 May 1;133(5):1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigati D. J., Myerson D., Leary J. J., Spalholz B., Travis S. Z., Fong C. K., Hsiung G. D., Ward D. C. Detection of viral genomes in cultured cells and paraffin-embedded tissue sections using biotin-labeled hybridization probes. Virology. 1983 Apr 15;126(1):32–50. doi: 10.1016/0042-6822(83)90460-9. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Craighead J. E. Pathogenesis of pulmonary cytomegalovirus infection in immunosuppressed mice. J Infect Dis. 1974 Jun;129(6):677–689. doi: 10.1093/infdis/129.6.677. [DOI] [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Welsh R. M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J Virol. 1984 Oct;52(1):119–128. doi: 10.1128/jvi.52.1.119-128.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceredig R., Dialynas D. P., Fitch F. W., MacDonald H. R. Precursors of T cell growth factor producing cells in the thymus: ontogeny, frequency, and quantitative recovery in a subpopulation of phenotypically mature thymocytes defined by monoclonal antibody GK-1.5. J Exp Med. 1983 Nov 1;158(5):1654–1671. doi: 10.1084/jem.158.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Ebeling A., Keil G. M., Knust E., Koszinowski U. H. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J Virol. 1983 Sep;47(3):421–433. doi: 10.1128/jvi.47.3.421-433.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers H. D., Glasebrook A. L., Sorenson G. D. Allogeneic tumor rejection induced by the intravenous injection of Lyt-2+ cytolytic T lymphocyte clones. J Exp Med. 1982 Oct 1;156(4):1280–1285. doi: 10.1084/jem.156.4.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Parker J., Estes J. D., Rongey R. W. Induction of disseminated virulent cytomegalovirus infection by immunosuppression of naturally chronically infected wild mice. Infect Immun. 1974 Oct;10(4):966–969. doi: 10.1128/iai.10.4.966-969.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Harris J. D., Traynor B., Ventura P., Peluso R., Brahic M. Visna DNA synthesis and the tempo of infection in vitro. Virology. 1982 Jun;119(2):399–410. doi: 10.1016/0042-6822(82)90099-x. [DOI] [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson J. B., Misra V., Mosmann T. R. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology. 1976 Jul 1;72(1):235–243. doi: 10.1016/0042-6822(76)90326-3. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Hämmerling U., Flaherty L. Qat-4 and Qat-5, new murine T-cell antigens governed by the Tla region and identified by monoclonal antibodies. J Exp Med. 1979 Jul 1;150(1):108–116. doi: 10.1084/jem.150.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C. Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infect Immun. 1978 Jul;21(1):275–280. doi: 10.1128/iai.21.1.275-280.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C., Shanley J. D., Stevens J. G. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol. 1977 Nov;37(2):419–423. doi: 10.1099/0022-1317-37-2-419. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Ebeling-Keil A., Koszinowski U. H. Temporal regulation of murine cytomegalovirus transcription and mapping of viral RNA synthesized at immediate early times after infection. J Virol. 1984 Jun;50(3):784–795. doi: 10.1128/jvi.50.3.784-795.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G. M., Fibi M. R., Koszinowski U. H. Characterization of the major immediate-early polypeptides encoded by murine cytomegalovirus. J Virol. 1985 May;54(2):422–428. doi: 10.1128/jvi.54.2.422-428.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen H. S., Feng M. F., Horohov D. W., Moore R. N., Rouse B. T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984 Apr;50(1):56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rouse R. V., Micklem H. S., Herzenberg L. A. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens. Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J Exp Med. 1980 Aug 1;152(2):280–295. doi: 10.1084/jem.152.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. L., Askonas B. A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981 Aug 1;154(2):225–234. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Armstrong J. A., Ho M. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature. 1977 Jun 23;267(5613):721–723. doi: 10.1038/267721a0. [DOI] [PubMed] [Google Scholar]
- Meyers J. D., Flournoy N., Thomas E. D. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis. 1982 Nov-Dec;4(6):1119–1132. doi: 10.1093/clinids/4.6.1119. [DOI] [PubMed] [Google Scholar]
- Mogensen S. C., Andersen H. K. Recovery of mice from herpes simplex virus type 2 hepatitis: adoptive transfer of recovery with immune spleen cells. Infect Immun. 1981 Sep;33(3):743–749. doi: 10.1128/iai.33.3.743-749.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Schwarz S. L., Rosenberg S. A. Adoptive immunotherapy of established pulmonary metastases with LAK cells and recombinant interleukin-2. Science. 1984 Sep 28;225(4669):1487–1489. doi: 10.1126/science.6332379. [DOI] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. I. Distinct maturation stages of cytolytic T lymphocytes constitute the cellular immune response during acute infection of mice with the murine cytomegalovirus. J Immunol. 1984 Jan;132(1):482–489. [PubMed] [Google Scholar]
- Reddehase M. J., Keil G. M., Koszinowski U. H. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur J Immunol. 1984 Jan;14(1):56–61. doi: 10.1002/eji.1830140111. [DOI] [PubMed] [Google Scholar]
- Reddehase M. J., Koszinowski U. H. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature. 1984 Nov 22;312(5992):369–371. doi: 10.1038/312369a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- SMITH M. G. Propagation of salivary gland virus of the mouse in tissue cultures. Proc Soc Exp Biol Med. 1954 Jul;86(3):435–440. doi: 10.3181/00379727-86-21123. [DOI] [PubMed] [Google Scholar]
- Sethi K. K., Omata Y., Schneweis K. E. Protection of mice from fatal herpes simplex virus type 1 infection by adoptive transfer of cloned virus-specific and H-2-restricted cytotoxic T lymphocytes. J Gen Virol. 1983 Feb;64(Pt 2):443–447. doi: 10.1099/0022-1317-64-2-443. [DOI] [PubMed] [Google Scholar]
- Shanley J. D., Pesanti E. L., Nugent K. M. The pathogenesis of pneumonitis due to murine cytomegalovirus. J Infect Dis. 1982 Sep;146(3):388–396. doi: 10.1093/infdis/146.3.388. [DOI] [PubMed] [Google Scholar]
- Starr S. E., Allison A. C. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977 Aug;17(2):458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L. Significance of Lyt phenotypes: Lyt2 antibodies block activities of T cells that recognize class 1 major histocompatibility complex antigens regardless of their function. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7101–7105. doi: 10.1073/pnas.78.11.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varho M., Lehmann Grube F., Simon M. M. Effector T lymphocytes in lymphocytic choriomeningitis virus-infected mice. Cytolytic activity of Lyt-23 spleen cells in vitro does not correlate with elimination of infectious virus from spleens. J Exp Med. 1981 Apr 1;153(4):992–997. doi: 10.1084/jem.153.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L., McKenzie I. F. Transfer of specific cytotoxic T lymphocytes protects mice inoculated with influenza virus. Nature. 1978 May 18;273(5659):238–239. doi: 10.1038/273238a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Althage A. Antiviral protection by virus-immune cytotoxic T cells: infected target cells are lysed before infectious virus progeny is assembled. J Exp Med. 1977 Mar 1;145(3):644–651. doi: 10.1084/jem.145.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]