Abstract
The membrane proteins of all regulated secretory organelles (RSOs) recycle after exocytosis. However, the recycling of those membrane proteins that are targeted to both dense core granules (DCGs) and synaptic-like microvesicles (SLMVs) has not been addressed. Since neuroendocrine cells contain both RSOs, and the recycling routes that lead to either organelle overlap, transfer between the two pools of membrane proteins could occur during recycling. We have previously demonstrated that a chimeric protein containing the cytosolic and transmembrane domains of P-selectin coupled to horseradish peroxidase is targeted to both the DCG and the SLMV in PC12 cells. Using this chimera, we have characterized secretagogue-induced traffic in PC12 cells. After stimulation, this chimeric protein traffics from DCGs to the cell surface, internalizes into transferrin receptor (TFnR)-positive endosomes and thence to a population of secretagogue-responsive SLMVs. We therefore find a secretagogue-dependent rise in levels of HRP within SLMVs. In addition, the levels within SLMVs of the endogenous membrane protein, synaptotagmin, as well as a green fluorescent protein-tagged version of vesicle-associated membrane protein (VAMP)/synaptobrevin, also show a secretagogue-dependent increase.
INTRODUCTION
Neuroendocrine cells have the ability to store both neuropeptides and transmitters for rapid release after external stimulation. Two major types of regulated secretory organelles (RSOs) are involved: the dense-core granules (DCGs), which contain peptides as well as classical transmitters, and the synaptic-like microvesicles (SLMVs), which contain only transmitters and which are closely related to the small synaptic vesicles (SSVs) of neurons. In neuroendocrine cells, comparable numbers of the two RSOs may be found, whereas the DCGs are the most common RSO of endocrine cells, and in neurons, SSVs dominate the secretory process.
The trafficking of membrane proteins found in the RSOs is complex, not least because the two organelles are formed and reformed (through recycling) in different ways. There are at least two routes for SLMV/SSV formation and recycling. SSVs/SLMVs can bud directly from the plasma membrane or an elaboration thereof (Takei et al., 1996; Schmidt et al., 1997). Alternatively SLMV proteins can pass from the plasma membrane to the SLMVs via the endosome (Clift-O’Grady et al., 1990; Régnier-Vigouroux et al., 1991; Desnos et al., 1995; Grote et al., 1995; Grote and Kelly, 1996; Faundez et al., 1997, 1998; Lichtenstein et al., 1998). Both routes exist within PC12 cells (Shi et al., 1998).
Unlike the SLMVs, the formation of DCGs is dependent on protein content. Newly synthesized proteins are incorporated into immature DCGs that bud from the trans-Golgi network (TGN; reviewed by Tooze and Stinchcombe, 1992; Arvan and Castle, 1998). The recycling of DCG membranes is much slower than that for SSVs, and it is not known whether multiple rounds of cycling can take place (Patzak and Winkler, 1986; Hunter and Phillips, 1989; von Grafenstein and Knight, 1992, 1993; Hurtley, 1993). Analyses of DCG recycling have shown internalization into peripheral endosomes as well as pericentriolar structures before reappearance in new granules (Patzak and Winkler, 1986; Phillips, 1987; von Grafenstein and Knight, 1992; Fischer von Mollard et al., 1994; Whalley et al., 1995).
In cells with both DCGs and SLMVs, differential release of peptides and small molecules has been demonstrated (Lundberg et al., 1986; Iverfeldt et al., 1989; Verhage et al., 1991; Franck et al., 1993). This depends on the two regulated secretory organelles having different responses to stimulation, which in turn implies their having a different membrane composition. The efficient sorting during traffic of RSO membrane proteins in the cell is therefore critical for the maintenance of this function.
Some proteins, such as synaptotagmin and vesicle-associated membrane protein (VAMP)/synaptobrevin, are found in both DCG and SLMV membranes (e.g., Lowe et al., 1988, Walch-Solimena et al., 1993; Papini et al., 1995); these proteins appear to be part of the requisite machinery for regulated exocytosis. The complexities of the trafficking of these important proteins are not well understood. In particular, the relationship between the two pools of such biorganellar proteins has not been explored. In this paper we examine whether proteins containing targeting signals for both RSOs could transfer from one RSO to the other, or whether the two pools of protein are kept separate within PC12 cells.
During stimulation, DCG membrane proteins transfer to the plasma membrane. From there, recycling back to the DCG will take place, with membrane proteins trafficking from the plasma membrane to an endosomal compartment before arrival at the TGN for incorporation into new granules. Recycling DCG proteins on their way back to the TGN will therefore traffic through the compartments (plasma membrane and endosomes) from which SLMVs are formed. In support of this scenario, Partoens et al. (1998) have recently demonstrated colocalization of synaptophysin (an SLMV marker) and dopamine β-hydroxylase (DBH; a DCG marker) in Rab5-positive endosomes of noradrenergic neurons after stimulation.
Since some membrane proteins within the DCG also contain targeting signals for SLMVs, they could transfer from DCGs to SLMV during postexocytic trafficking. Movement between the two membrane populations would have important consequences for the cell. Uncontrolled mixing would result in the loss of the separate character of the two RSOs, affecting physiological processes such as differential release.
We have taken a quantitative approach to the analysis of poststimulation trafficking, primarily using a chimeric protein composed of the cytoplasmic and transmembrane domain of P-selectin and the enzymatic marker HRP (ssHRPP-selectin). We have previously demonstrated that this chimera is targeted to both DCGs and SLMVs in PC12 cells (Norcott et al., 1996), thereby providing a model protein with targeting signals for both RSOs.
Using PC12 cells expressing ssHRPP-selectin we demonstrate that, after secretagogue stimulation, ssHRPP-selectin moves out of the DCG and passes over the cell surface and through the transferrin-positive endosome en route to the SLMV. Further, both VAMP/synaptobrevin and synaptotagmin levels in the SLMVs increase in a secretagogue-dependent manner.
MATERIALS AND METHODS
The figures of this paper show representative data sets from single experiments. Where normalization for differences in levels of expression between experiments has been performed to allow interexperimental variation to be determined, the results of such quantifications are in the text.
Cell Culture, Transfection, and Constructs
PC12 cells were grown as described previously (Cramer and Cutler, 1992). One day before transfection the cells were plated to ∼45% confluency. The cells were then transfected as described previously (Cramer and Cutler, 1992; Norcott et al., 1996). ssHRPP-selectin was described previously by Norcott et al. (1996), and ssHRPP-selectinKCPL was described by Blagoveshchenskaya et al. (1998a). The transferrin receptor (TfnR)-HRP construct was described by Stinchcombe et al. (1995). The green fluorescent protein (GFP)-VAMP construct was a gift of Roberto Solari and Nicki Thompson (Glaxo-Wellcome, Stevenage, UK).
Antibodies
Rabbit anti-synaptophysin was prepared as described previously (Cutler and Cramer, 1990). Rabbit anti-synaptotagmin was a kind gift of Gary E. Dean (Cincinnati, OH), and rabbit anti-GFP was a kind gift from David T. Shima (Imperial Cancer Research Fund, London). Monoclonal anti-HRP antibody 2H11 was purchased from Advanced Immunochemicals (Long Beach, CA). 2H11 and Tfn were iodinated using iodo-gen as described elsewhere (Blagoveshchenskaya et al., 1998a).
Glycerol Gradient for SLMV Isolation
PC12 cells were stimulated as described below at various times post transfection. They were then either chased for various times or immediately chilled, homogenized, and fractionated as described by Kelly and co-workers (Desnos et al., 1995) and subsequently modified (Norcott et al., 1996).
Ficoll Gradient Fractionation for DCG Enrichment
After transfection cells were grown under normal conditions for the indicated time. The cells were stimulated as described below, allowed to recover for the indicated time, quickly chilled, homogenized, and then fractionated on 1–16% Ficoll as previously described (Cramer and Cutler, 1992; Norcott et al., 1996).
Endosomal Ficoll Gradient Fractionation
The double-gradient system for endosomal enrichment used is a modification of that described elsewhere (Blagoveshchenskaya et al., 1998a). Cells were loaded with 125I-Tfn, stimulated, and either immediately placed on ice, rinsed, homogenized, and fractionated on the first (1–16%) Ficoll gradient, or chased for 30 min and then chilled and fractionated. Unstimulated controls were processed in parallel. Fractions enriched in 125I-Tfn were then pooled and subjected to a second (3–16%) Ficoll gradient centrifugation and fractions were assayed for the presence of 125I-Tfn and HRP.
HRP Assay
HRP assays were carried out as previously described (Norcott et al., 1996). All graphs show data that have been normalized by HRP activity in the cell lysate.
Secretagogue Stimulation
The DCGs were labeled by incubating the cells for 2 h with 0.5 μCi/ml 3H-dopamine (Amersham International, Amersham, UK) in DMEM. The external dopamine was then removed by three washes with DMEM, and the cells were stimulated for 5 min (unless otherwise indicated) with 10 mM carbamylcholine (carbachol) in DMEM. To determine the percent of 3H-dopamine secreted, the amount of 3H in the media and the amount of 3H in the homogenate were added together and set at 100%. The amount of 3H in the media relative to the total was then determined.
Stimulation was followed by two washes with DMEM and a chase in normal growth media for the indicated times, and then cells were fractionated. If the cells were to be stimulated at a lower temperature, they were preincubated at that temperature for 20 min before stimulation.
To determine the amount of ssHRPP-selectin in the SLMV peak fractions in stimulated or mock-stimulated cells (e.g., Figure 9B), the area under the peak was determined by adding the HRP activity (after subtracting background) in the fractions of the peak and dividing by the activity in the homogenate. The area of the peak in stimulated cells was then divided by the area (from the same fractions) in unstimulated cells to give the fold increase.
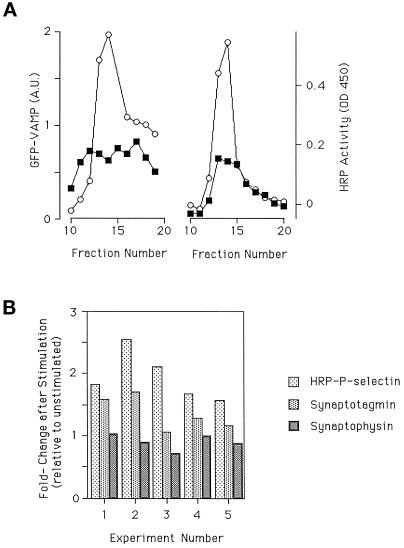
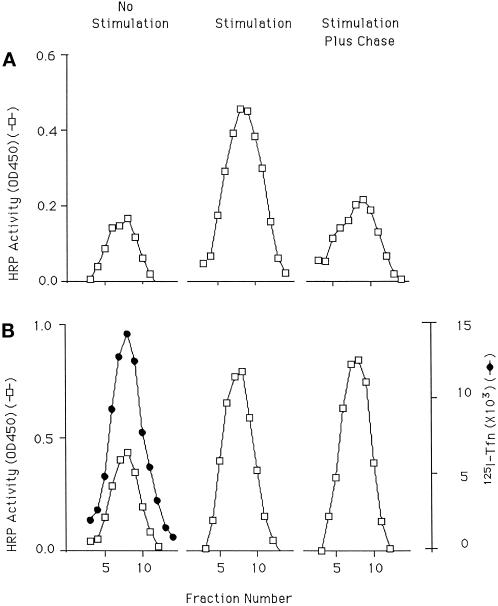
Figure 9.
Trafficking of endogenous proteins after stimulation. (A) One each of paired dishes of cells expressing GFP-VAMP or ssHRPP-selectin were kept as controls or stimulated and then chased and fractionated on glycerol gradients. Fractions were collected and assayed for the presence of GFP-VAMP (by quantitative immunoblotting using antibodies to GFP) or HRP (determined by enzyme activity). ▪, unstimulated; ○, stimulated. (B) Cells expressing ssHRPP-selectin were stimulated, chased and then fractionated on glycerol gradients. The region of the gradient containing the SLMVs was determined by assaying for HRP activity. Those fractions that contained SLMVs were subjected to quantitative immunoblotting using antibodies to synaptophysin or synaptotagmin. Levels of expression in the SLMV peak without stimulation were set to 1 (control). Each group represents an individual experiment (data from 5 individual experiments are shown).
To quantify the HRP activity in the DCG-containing fractions, the region of the gradient containing DCGs was identified by the distribution of preloaded 3H-dopamine. The HRP activity in the peak was then determined as described for SLMVs. The HRP activity in the DCG peak before stimulation was then divided by that after stimulation to give the fold decrease. To compare different experiments it was necessary to compensate for the variation in level of expression. We have therefore determined the percent change (in the DCG or SLMV peak) with and without stimulation in each experiment and used that value to compare different experiments.
Anti-HRP Antibody Uptake
PC12 cells expressing the ssHRPP-selectin chimera were grown on 10-cm dishes and analyzed 8–9 d posttransfection. The cells were stimulated or mock stimulated with carbachol for 10 min at 37°C in the growth medium containing 0.5 μg/ml 125I-2H11 (anti-HRP antibody), washed, and chased for an additional 30 min in fresh medium. Cells were then fractionated in a glycerol gradient as described above, and the fractions were assayed for the presence of 125I on a γ-counter.
To preload cells with antibody, cells were incubated in the presence of a 100-fold excess of cold antibody for 3 h under normal culture conditions. After the preload, free antibody was removed by extensive washing. To examine the stimulation-dependent internalization of iodinated antibody, the cells were stimulated (or mock stimulated) in the presence of iodinated antibody 125I-2H11 as described above. After stimulation, the cells were washed, allowed to recover, and fractionated on a glycerol gradient.
Immunoblotting
To examine the impact of secretagogue stimulation on the subcellular localization of GFP-VAMP, cells transfected with the GFP-VAMP cDNA were stimulated or left as controls, chased for 30 min, and then fractionated on glycerol gradients. The fractions were analyzed by SDS-PAGE, transferred to nitrocellulose using standard conditions (Harlow and Lane, 1988), and probed with antibody to GFP. The immunoreactivity was quantified using a Bio-Rad GS-250 Molecular Imager (Bio-Rad, Richmond, CA). The value obtained from each fraction was normalized by division with the value obtained from the total cell lysate, run in parallel.
To determine the effects of secretagogue stimulation on the localization of endogenous SLMV proteins, cells transfected with ssHRPP-selectin were stimulated and fractionated on glycerol gradients. HRP activity was assayed across the gradient to determine the SLMV peak. Those fractions containing the SLMVs were separated by SDS-PAGE and immunoblotted for synaptotagmin and synaptophysin. The immunoblots were quantified as above. The values obtained for each fraction of the peak were added and compared with the amount of protein in the unstimulated SLMV peak, which was set as 100%.
RESULTS
Postexocytic traffic of molecules that are found in both RSOs may be very complex. Both organelles will respond to secretagogue by fusing with the plasma membrane, after which internalization of the membrane proteins will take place, leading to recycling back to the organelle of origin, or to cross-targeting to the other RSO. In either case, the route could be indirect, involving multiple steps. To follow this trafficking, we have used a chimera between P-selectin and HRP in which the lumenal part of this type-1 membrane protein has been replaced with the active enzyme to allow for easy, sensitive, and quantitative detection of the protein. The cytoplasmic tail of P-selectin has been shown to contain sequences needed for targeting to both SLMVs and DCGs as well as a lysosomal targeting signal (Blagoveshchenskaya et al., 1999). The transmembrane domain has also been shown to enhance DCG targeting (Fleming et al., 1998).
After secretagogue action, the ssHRPP-selectin will reach the plasma membrane. From here, internalization can lead to a variety of destinations. In this initial analysis of poststimulation trafficking, we have assayed for appearance in the SLMVs as one of the first destinations that will be reached once secretion is triggered.
We have used the advantages of transient transfection in a novel way to simplify the trafficking that occurs after secretagogue stimulation of PC12 cells expressing ssHRPP-selectin. Since between 30 and 80% of the PC12 cells transiently express ssHRPP-selectin after electroporation (assayed by immunofluorescence; our unpublished data) this approach does not give data relating only to a small subset of the cells. Moreover, the consistency of the data obtained in this and other studies (Blagoveshchenskaya et al. 1998a,b, 1999) supports our use of this approach. In order to follow postexocytic traffic, it is important to distinguish between DCG- and SLMV-derived pools of chimera. We have been able to do this by exploiting the differential turnover of the two RSOs. In the absence of stimulation the t1/2 for SLMVs has been measured at ∼30 min (Faundez et al., 1997). Examination of the turnover of stably expressed P-selectin within PC12 cells (Green et al., 1994) revealed that whereas material in light fractions (including the SLMVs) is rapidly degraded, material in dense fractions in which the DCGs are located is more stable. In confirmation of this, we found that after transient expression of our ssHRPP-selectin chimera, the HRP activity in DCGs was stable for many days, while that in SLMVs declined relatively rapidly (Norcott et al., 1996). Thus, with increasing time after transfection, an increasing fraction of the HRP activity within the cell will be in the DCGs. By using cells under such conditions as starting material for the examination of postexocytic trafficking, we have significantly simplified the problem.
PC12 cells were transfected with ssHRPP-selectin. After 3 d, one of a pair of parallel dishes of cells was stimulated to secrete with carbamylcholine and chased for 30 min. Both dishes were then placed on ice, a postnuclear homogenate was prepared, and the standard glycerol gradient fractionation was employed to quantify levels of HRP activity in SLMVs for stimulated and mock-stimulated cells (Desnos et al., 1995; Norcott et al., 1996). In this as in all other experiments, levels of HRP activity are normalized for levels of expression. Figure 1A shows a significant increase in the levels of ssHRPP-selectin in the SLMV peak in stimulated as compared with unstimulated cells, suggesting that ssHRPP-selectin has appeared in the SLMVs in response to secretagogue stimulation. When a similar experiment was carried out 7 d after transfection (Figure 1B), the amount of HRP activity in the SLMV peak in the absence of stimulation was much lower, as predicted, but secretagogue action again leads to the appearance of a large SLMV peak.
Figure 1.
Appearance of HRP chimeras in SLMVs after secretagogue stimulation. Pairs of 10-cm dishes were plated with PC12 cells transfected with ssHRPP-selectin (A and B) or TfnR-HRP (C) and incubated for 3 (A) or 7 d (B and C). One of each pair was then stimulated to secrete with carbamylcholine for 5 min, chased for 30 min, and then fractionated on a glycerol gradient (MATERIALS AND METHODS). The graph shows the amount of HRP activity in gradient fractions (MATERIALS AND METHODS). All points are the mean of triplicate assays for that fraction. Error bars indicate the SD for the intraexperimental variation. Note that in this and most subsequent figures, the variability of the HRP assay is so low that no error bars can be seen at the scale of reproduction used here. Interexperimental variation is detailed in the text. The effectiveness of stimulation was monitored in all cases by preloading with 3H-dopamine and counting media and cell samples. Fraction 1 is at the top of all gradients. □, Unstimulated; ♦, stimulated.
We find that each set of transient transfections has a variable rate at which this differential decay occurs. Of the subsequent experiments shown in this article, one has no presecretagogue peak, whereas the others do have a preexisting SLMV peak in the absence of stimulation. In general, at least 7 d of growth after transfection is required for no HRP activity to be found in the SLMVs in the absence of stimulation.
One trivial explanation for the secretagogue-dependent increase in HRP activity within SLMV would be that some combination of overexpression by transient transfection plus secretagogue action coupled to the sensitivity of detection afforded by using HRP chimeras is revealing a nonspecific overspill into the SLMVs. To control for this, we have examined the behavior of a chimera between the transferrin receptor and HRP, in which HRP replaces the lumenal domain of the receptor (Stinchcombe et al. 1995). Transient overexpression of this chimera, followed by determining levels of HRP activity in the SLMVs from cells 7 d posttransfection that have been treated with secretagogue or left as controls (Figure 1C), shows that in neither case have we caused a movement of chimera from its normal itinerary of cycling between the endosome and the plasma membrane into the SLMVs. This strongly suggests that we have a signal-dependent and -specific phenomenon.
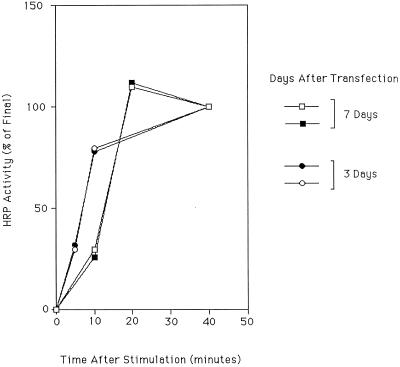
To further characterize the phenomenon, we determined the rate at which appearance of HRP activity in SLMVs occurs. Cells expressing ssHRPP-selectin were stimulated with secretagogue and then chased for various times, after which they were fractionated. The results show (Figure 2) that stimulation is followed by a rapid increase in the amount of ssHRPP-selectin in the SLMV, the rate of which slows toward a plateau within ∼20 min after stimulation (Figure 2). This experiment has been done on cells 3 or 7 d after transfection. While there is a subtle but reproducible difference between cells examined 3 or 7 d after transfection, the movement of ssHRPP-selectin into the SLMV after stimulation is a fast, relatively synchronous process at both times, and the slopes between the early lag phase and the final plateau are very similar in all experiments.
Figure 2.
Time course of appearance of HRP activity in SLMVs. PC12 cells transfected to express ssHRPP-selectin were cultured for 3 (●, ○) or 7 d (□, ▪). Dishes were then kept as controls or stimulated for 5 min and then chased for a further 5, 10, and 40 or 10, 20, and 40 min before fractionation on glycerol gradients. The HRP activity in the SLMV peak at each time point was then calculated, the amount of HRP activity in the SLMV peak at 40 min was set to 100%, and the amount of HRP activity in the SLMV peak before stimulation was subtracted. Each data point represents the mean of triplicate assays for that peak. Data from four separate experiments are shown.
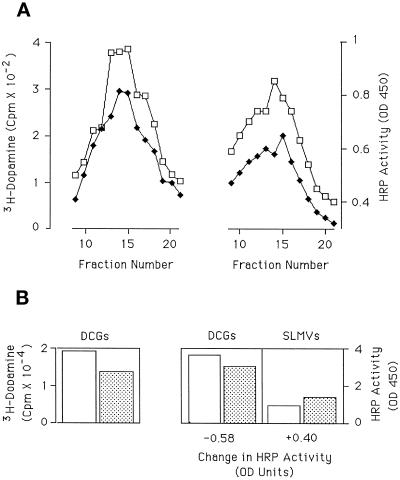
The most likely source of the HRP chimera that is moving into the SLMVs after stimulation is the DCGs, since these would provide a secretagogue-responsive stable pool of membrane within PC12 cells, consistent with both the retention of the phenomenon 7 d after transfection and with the known targeting behavior of the chimera. To monitor the effects of stimulation on granules, we incubated cells with 3H-dopamine for 2 h before the addition of secretagogue in order to load the granules. This was used to determine both the effectiveness of secretagogue stimulation and the distribution of DCGs in subcellular fractionation. After secretagogue stimulation and a subsequent 30-min chase, PC12 cells expressing ssHRPP-selectin were fractionated to enrich for DCGs. Scintillation counting of cell extracts and media samples demonstrate that 37% (±11%; n = 3) of intracellular 3H-dopamine was released into the bathing medium over the 5-min secretagogue stimulation, as compared with 9% (±13%; n = 3) released in the absence of stimulation. Consistent with the loss of 3H-dopamine from the cell homogenates, fractionation revealed a 41% (±8%, n = 3) decrease of 3H-dopamine in the DCG peak after secretagogue-stimulation (Figure 3A). This is in line with work from other groups indicating that ∼30% of the DCGs release their content by exocytosis in response to stimulation (Schweitzer and Kelly, 1985). The ssHRPP-selectin HRP activity in the DCG peaks falls by 21% (±4%, n = 3). When the appearance of ssHRPP-selectin in the SLMV after stimulation is analyzed in parallel to its disappearance from the DCG, at least 67% (±9%; n = 3) of the HRP activity lost from the DCGs has moved to the SLMV (as illustrated for the data set in Figure 3A by Figure 3B).
Figure 3.
Disappearance of HRP activity from DCGs after secretagogue stimulation. PC12 cells transfected to express ssHRPP-selectin were plated onto two pairs of 10-cm dishes, and preloaded with 3H-dopamine. Stimulation of one pair of dishes for 5 min, and incubation for 30 min was then carried out. The cells were then fractionated, one each of stimulated and unstimulated, onto a glycerol gradient to determine appearance in SLMVs and, one each of stimulated and unstimulated, onto a Ficoll gradient (Norcott et al., 1996, and MATERIALS AND METHODS) to monitor disappearance from DCGs. (A) DCG peaks from Ficoll gradients showing 3H-dopamine (lefthand panel) and HRP activity (righthand panel). Each data point represents the mean of triplicate assays for that fraction; error bars indicate the SD within the triplicate. Interexperimental variation is detailed in the text. □, Unstimulated; ♦, stimulated. (B) Quantification of the change in 3H-dopamine (lefthand panel) and HRP activity (righthand panel) in the DCG peaks (DCGs) and in the SLMVs (SLMVs). The latter was determined from the parallel glycerol gradient (data not shown). The fall or rise in the total HRP activity in the DCGs and the SLMVs is shown under the relevant panel. Interexperimental variation is detailed in the text. Clear column, unstimulated; hatched column, stimulated.
The fall in HRP activity within DCGs after stimulation is smaller than that seen for 3H-dopamine. We suspect that this may reflect the selective labeling of less-easily-stimulated older granules with HRP, as compared with the uptake of 3H-dopamine by all granules, because this experiment was done 7 d after transfection. These data, coupled to the secretagogue dependence of the phenomenon, strongly suggest that the source of material arriving in the SLMVs is the DCG.
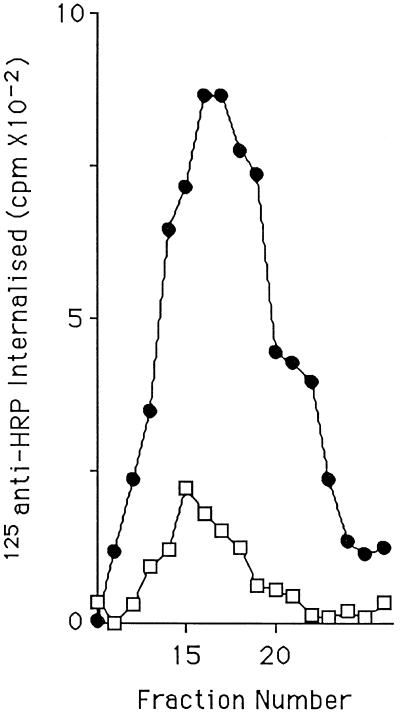
If the HRP activity appearing in the SLMVs after stimulation is indeed originating in the DCGs, then the transfer of material from one organelle to the other will have predictable characteristics. Secretagogue stimulation causes fusion of RSOs with the plasma membrane. If the chimera passes from the DCG to the SLMV, then it must transiently appear on the plasma membrane at DCG exocytosis. To verify that the ssHRPP-selectin appearing in the SLMV after stimulation has appeared at the plasma membrane, cells transiently transfected to express ssHRPP-selectin were stimulated in the presence of antibodies to HRP that had been radioiodinated. The antibodies that we used recognize the HRP domain of ssHRPP-selectin, which is extracellular when RSOs fuse with the plasma membrane. As seen in Figure 4, the anti-HRP antibody accumulates in the SLMV peak in a stimulation-dependent manner, indicating that ssHRPP-selectin does pass over the cell surface.
Figure 4.
Stimulation-dependent labeling of SLMVs with 125I-anti-HRP. PC12 cells expressing ssHRPP-selectin were stimulated or mock stimulated in the presence of iodinated anti-HRP. After stimulation the cells were chased for 30 min and then fractionated in parallel on glycerol gradients. Levels of 125I in individual gradient fractions were then determined using a γ-counter. □, Unstimulated; ●, stimulated.
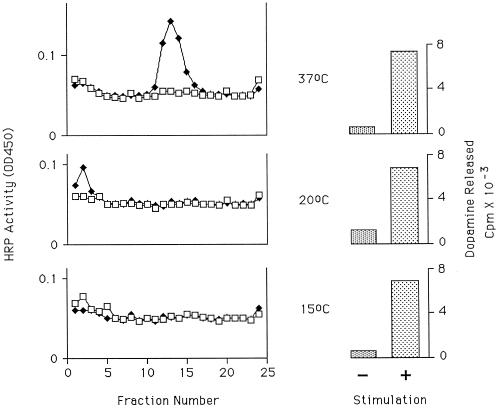
After appearance on the plasma membrane, there is more than one route that the chimera could take to the SLMV. Both a direct route involving budding from the plasma membrane or an invagination thereof (Takei et al., 1996; Schmidt et al., 1997) and an indirect route via endosomal intermediate(s) have been described (see INTRODUCTION). Previous experiments (Norcott et al., 1996; Blagoveshchenskaya et al., 1999) with ssHRPP-selectin in PC12 cells suggested the involvement of endosomes in SLMV biogenesis. Also, in primary noradrenergic cells, SLMV and DCG markers colocalize within endosomes after stimulation (Partoens et al., 1998). Moreover, in PC12 cells the level of ssHRPP-selectin in endosome-containing fractions of the 1–16% Ficoll gradients used for a general survey of organelles (Norcott et al., 1996) increases by 6% (±4%, n = 3) after stimulation (not shown). This suggested an endosomal intermediate in the poststimulation traffic of this protein. Many experiments have shown that reduction of the temperature to 20°C blocks transfer of ligand or pseudoligand from the early or TfnR-rich endosome to more distal parts of the endocytic system (Salzman and Maxfield, 1989; Futter et al., 1996). Further, Kelly and co-workers have shown that lowering the temperature to 15°C prevents the formation of SLMVs from an endosomal compartment (Desnos et al., 1995). We therefore determined the effect of temperature reduction on the transfer of ssHRPP-selectin into SLMVs. Cells were stimulated and chased at 37, 20, or 15°C and then fractionated (Figure 5). Stimulation was effective at all three temperatures as judged by 3H-dopamine release. Despite this, at both 15 and 20°C there is no appearance of a peak of HRP activity in the SLMV-containing fractions, indicating that both temperatures inhibit the transfer into SLMVs.
Figure 5.
Effects of temperature on appearance of HRP activity in SLMVs. PC12 cells transfected to express ssHRPP-selectin were plated onto three pairs of 10-cm dishes and incubated for 3 d. Cells were cultured then preloaded with 3H-dopamine, and one pair was placed at 15°C, one pair at 20°C, and the third pair remained at 37°C. One of each pair was then stimulated for 5 min and then chased for 30 min. The other dish was mock stimulated and left as control. The cells were then fractionated on glycerol gradients, and HRP activity in each fraction was determined in triplicate. Error bars indicate the SD within the triplicate. The mean of activity in each fraction from stimulated (♦) or unstimulated (□) cells are shown. To monitor for effectiveness of secretagogue action, 3H-dopamine in media samples was counted for stimulated (+) and unstimulated (−) samples. The top panels show data from cells treated at 37°C, the middle panels show the data from cells treated at 20°C, and the bottom panels show data from cells treated at 15°C.
If there is an endosomal intermediate involved in the secretagogue-dependent trafficking of ssHRPP-selectin, it should be possible to detect a transient increase in the level of HRP within endosomes as the chimera passes from the plasma membrane to the SLMVs. We have developed a gradient system specifically designed to enrich for Tfn-containing endosomes, which we used to determine whether this is the case. As a control we analyzed in parallel the behavior of a mutant ssHRPP-selectin chimera, ssHRPP-selectinKCPL, in which the four amino acids of the P-selectin cytoplasmic domain indicated are replaced with alanine, is efficiently targeted to the DCGs but does not enter the SLMVs, accumulating instead within Tfn-containing endosomes (Blagoveshchenskaya et al., 1998b, 1999). We reasoned that both chimeras should show a similar secretagogue-induced transfer from granules into endosomes, but that the mutant should remain within endosomes while the ssHRPP-selectin exits toward the SLMVs.
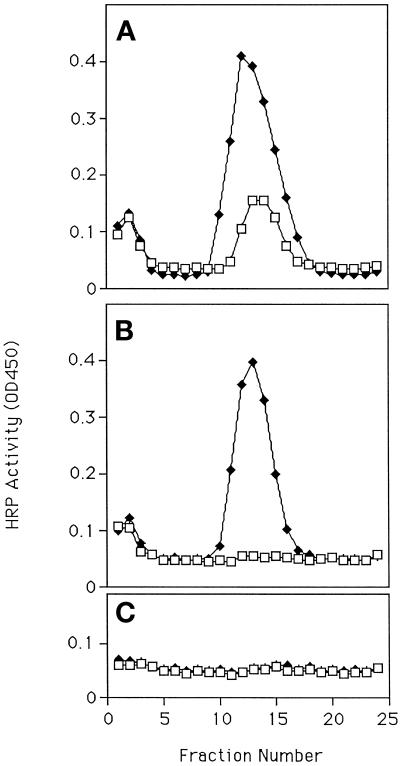
The recycling endosomes of PC12 cells transfected with either ssHRPP-selectin or ssHRPP-selectinKCPL were prelabeled with 125I-Tfn. The cells were then stimulated and harvested with or without a recovery period and then fractionated along with unstimulated control cells. The mutant protein (Figure 6B) shows a larger preexisting endosomal pool than the wild-type chimera (Figure 6A) in the absence of stimulation, as predicted for a mutation that prevents exit from the TfnR-rich endosome. However, cells expressing the wild-type ssHRPP-selectin (Figure 6A) or ssHRPP-selectinKCPL (Figure 6B) show a similar striking increase in endosomal HRP activity immediately after stimulation. The data clearly show that after the chase period, ssHRPP-selectin leaves the endosome while ssHRPP-selectinKCPL remains.
Figure 6.
Poststimulation trafficking involves an endosomal intermediate. PC12 cells expressing either ssHRPP-selectin or ssHRPP-selectinKCPL were preloaded with 125I-Tfn and were stimulated and chased. Cells were then fractionated on a 1–16% Ficoll gradient. Those fractions enriched for endosomes, as judged by iodinated transferrin, were pooled and run on a second 7–25% Ficoll gradient from which fractions were again collected and assayed for the presence of HRP activity as well as 125I-Tfn. □, HRP activity; ●, levels of 125I radioactivity, shown for one representative gradient. (A) ssHRPP-selectin. (B) ssHRPP-selectinKCPL.
Our data are consistent with a model where ssHRPP-selectin present within the DCGs transiently appears on the plasma membrane after secretagogue action, and then passes through Tfn+ endosomes before arriving in SLMVs. However, it is possible that the DCGs are not the source of the chimera appearing in the SLMVs, but that the pool of chimera within the endosome (Figure 6A) could instead be the immediate precursor. To test this hypothesis, we exploited the fact that the Tfn+ endosomal membranes constitutively cycle to the plasma membrane, whereas the DCGs are stable in the absence of secretagogue. This allows for differential labeling of the two populations by anti-HRP antibodies.
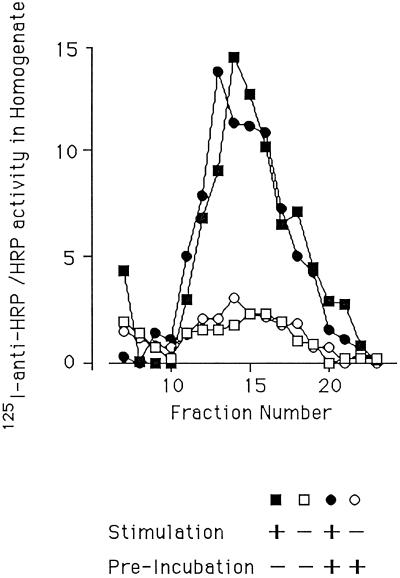
Cells were transiently transfected to express ssHRPP-selectin and then treated/mock treated with secretagogue and chased in the presence of 125I-labeled anti-HRP antibodies in the bathing medium. The dishes were placed on ice, a postnuclear supernatant was prepared, and the samples were fractionated on a glycerol gradient. This allowed us to monitor the secretagogue-dependent appearance within SLMVs of chimera moving to the cell surface from the granules as well as monitoring any contribution from the recycling endosomes. In addition, a second pair of dishes were preincubated for 3 h with a 100-fold excess of cold antibody to block all binding sites on the constitutively recycling endosomal chimera before stimulation in the presence of labeled antibodies and processing as above. This allowed us to monitor only that chimera reaching the surface from the granules. If the endosomal chimera provided a significant proportion of the ssHRPP-selectin arriving in the SLMVs, then the preincubation should have reduced the secretagogue-dependent appearance of antibody within the SLMVs. On the other hand, if the chimera appearing in the SLMVs after stimulation had been sequestered within the DCGs away from the blocking antibodies, then it would be able to bind the iodinated antibody, and preincubation should have no effect. Figure 7 shows a large secretagogue-dependent increase in anti-HRP within the SLMVs, which is unaffected by the preincubation. This serves to confirm that the endosomal pool of chimera is not the source of HRP activity that appears in the SLMVs after stimulation.
Figure 7.
The endosome is not the donor compartment. PC12 cells expressing ssHRPP-selectin were incubated in the presence or absence of a 100-fold excess of HRP antibody for 3 h (preincubatedî). The cells were then stimulated or mock stimulated and chased in the presence of radioiodinated anti-HRP, and postnuclear supernatants were fractionated on glycerol gradients. Levels of 125I in individual gradient fractions were then determined using a γ-counter and presented as counts per min normalized by levels of HRP activity in cell homogenates. □, Unstimulated with no preincubation; ▪, stimulated with no preincubation; ○, unstimulated and preincubated; ●, stimulated and preincubated
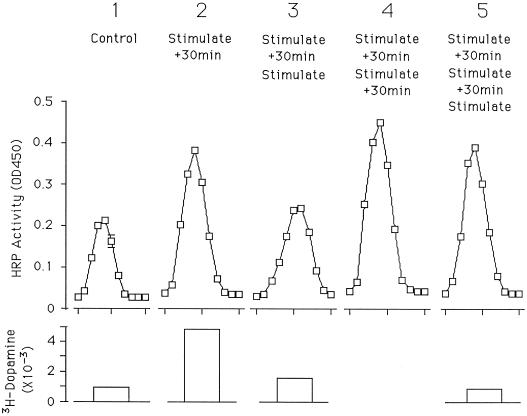
To demonstrate that the compartment into which the ssHRPP-selectin was transferring after stimulation is a bona fide RSO, we transfected PC12 cells with ssHRPP-selectin and carried out a multiple stimulation experiment. Cells were stimulated once, twice, or three times with and without subsequent incubation before fractionation. As shown in Figure 8, after a first stimulation and chase, HRP activity within the SLMV peak rises as expected (compare peaks 1 and 2). If the cells are stimulated again and fractionation is carried out immediately after the second stimulation (peak 3), the ssHRPP-selectin in the peak falls by 70% (± 2%; n = 3), indicating that this is a secretagogue-responsive compartment and that, like granules, not all SLMVs fuse at each stimulation. Presumably the disappearance of HRP activity from the SLMV peak is accompanied by its appearance on the plasma membrane or in other compartments of the recycling route. A detailed examination of SLMV functioning should now be possible with this system.
Figure 8.
Effects of multiple stimulations on HRP activity in SLMVs. Five 10-cm dishes were plated with PC12 cells transfected to express ssHRPP-selectin, left for 3 d, and then incubated with 3H-dopamine for 2 h. One dish was left unstimulated (control; panel 1), one dish was stimulated for 5 min and then left for 30 min (stimulate, + 30 min; panel 2), one dish was treated as in panel 2 and then stimulated again for 5 min (stimulate, + 30 min, stimulate; panel 3), one dish was treated as in panel 3 and then allowed to recover for 30 min (simulate, + 30 min, stimulate, + 30 min; panel 4), and one dish was treated as in panel 4 and then stimulated for a third time for 5 min (stimulate, + 30 min, stimulate, + 30 min, stimulate; panel 5). Each dish was then fractionated and HRP activity in fractions from a glycerol gradient was measured in triplicate. Means of determinations are shown; error bars indicate the SD for that experiment. Interexperimental variation is detailed in the text. The bar graph under each panel indicates the amount of 3H-dopamine released during the last 5-min stimulation of the procedure carried out on that dish.
If the cells are then chased for a further 30 min, the HRP activity in the SLMV peak rises again, but to a level that is not much higher than that found after the first stimulation and chase (compare peaks 2 and 4). Because peak 4 is only slightly larger than peak 2, the majority of the increase between peak 3 and peak 4 probably reflects recycling from the SLMV rather than transfer from granules, given that few granules have responded to the second stimulation (see lower panel). This data set is therefore also consistent with the DCGs being the origin of the ssHRPP-selectin that is arriving in the SLMVs in response to secretagogue. The small response of the SLMV population to a third stimulation, as reflected by the difference in HRP activity in SLMVs between panels 4 and 5, suggests that the SLMVs as well as the DCGs are showing a diminished response to repetitive applications of carbamylcholine.
ssHRPP-selectin is not found endogenously in PC12 cells. It is therefore possible that the route we describe is not the route taken by endogenous proteins but an anomaly. DePotter’s group (Partoens et al., 1998) has demonstrated the intermingling of at least one endogenous DCG protein (DBH) and one endogenous SLMV protein (synaptophysin) in rab5-positive endosomes after stimulation. This indicates that at least part of the pathway is followed by endogenous proteins. To verify that transfer occurs with proteins normally found in PC12 cells, we examined the poststimulation traffic of VAMP.
VAMP is normally found in both DCGs and SLMVs in PC12 cells (Papini et al., 1995). To examine the behavior of VAMP under the conditions used to characterize ssHRPP-selectin trafficking, we followed the trafficking of VAMP tagged with green fluorescent protein (GFP) after transfection. PC12 cells transiently transfected with either GFP-VAMP or ssHRPP-selectin were stimulated to secrete, and then chased and fractionated as described above. The amount of GFP in the SLMV peak with and without stimulation (normalized for expression) was then quantified by immunoblotting and compared with HRP activity from ssHRPP-selectin processed in parallel. As seen in Figure 9A, the movement of VAMP-GFP into the DCG after stimulation is comparable to that of ssHRPP-selectin, suggesting that endogenous proteins do utilize this pathway.
To ensure that the phenomenon of transfer is not the result of expression by transfection, we have also followed the behavior of an endogenously expressed protein. Synaptotagmin is another example of a membrane protein found in both RSOs (Elferink et al., 1993). Experiments in which synaptotagmin and ssHRPP-selectin were analyzed together (Figure 9B) indicate that like ssHRPP-selectin and GFP-VAMP, levels of endogenous synaptotagmin in the SLMVs increase after stimulation. Clearly, the fold increase seen with synaptotagmin after stimulation is consistently less than that found for ssHRPP-selectin and GFP-VAMP. Presumably, this is because we are monitoring an endogenous protein for which there is a larger preexisting pool of material within the SLMVs than is the case for a heterologously expressed protein. The difference in fold increase between the transfected and endogenous proteins serves to illustrate the way in which a transient transfection approach has helped in the analysis of postexocytic trafficking. Synaptophysin, in contrast to the aforementioned proteins, is found in the SLMV but not in the DCG (Jahn et al., 1985; Weidenmann and Franke, 1985; Rehm et al., 1986). We would predict, therefore, that there would be no increase in levels of synaptophysin in the SLMV after stimulation. In support of our hypothesis, synaptophysin levels are essentially unchanged in response to stimulation (Figure 9B). It is worth noting that there is also a large pool of synaptophysin within endosomes in PC12 cells. The failure to see a rise in levels of synaptophysin within the SLMV after stimulation therefore also confirms that there is no secretagogue-triggered spillover from the endosomes into the SLMVs.
DISCUSSION
This work provides a detailed examination of the poststimulation traffic of individual proteins that are found in both RSOs in a system where both organelles can be monitored. Our experimental design, exploiting transient transfection in a novel way, has allowed us to selectively track a protein originating in the DCG rather than the SLMV or the secretory pathway (as for a newly synthesized component). We have discovered that upon stimulation, ssHRPP-selectin appears at the cell surface followed by a rapid and transient increase of HRP activity within Tfn-positive endosomes (Figure 6), and then finally appears within secretagogue-responsive, i.e., functionally active SLMVs. Our data characterize a novel trafficking pathway and reveal the potential for postexocytic transfer from one RSO (the DCG) to another (the SLMV) in PC12 cells.
The secretagogue dependence of the appearance of ssHRPP-selectin in the SLMVs strongly suggests that the organelle from whence the secretagogue-dependent increase in SLMV-located chimera arises is the DCG. This is also consistent with the secretagogue-dependent surface exposure of the chimera that appears in the SLMVs (Figure 4), as well as its transient passage through endosomes (Figure 6). The fact that the increase in SLMV-associated HRP activity is 67% of the HRP activity that disappears from DCGs after stimulation also supports the idea of the DCG as the donor organelle. This is supported further by the correlation between the levels of dopamine release (i.e., granule exocytosis) and the magnitude of the rise in HRP activity within the SLMVs (Figure 3).
Three observations argue against the endosome as the organelle from which the chimera is transferred. First, an endosomal marker, the TfnR-HRP chimera, does not appear in the SLMVs after stimulation (Figure 1C), showing that appearance in the SLMVs is not nonspecific, but requires appropriate targeting signals. Second, synaptophysin does not increase within the SLMV after stimulation (Figure 9) even though it must contain SLMV-targeting signal(s) and PC12 cells contain a significant endosomal pool of synaptophysin (Cameron et al., 1991; Lah and Burry, 1993). Third, our direct examination of the contribution from the consitutively recycling endosomally located ssHRPP-selectin to the SLMV peak after stimulation (Figure 7) confirms that this population of ssHRPP-selectin does not play a significant role in this phenomenon.
The exact route taken by RSO membranes after fusion with the plasma membrane is not clear. Historically, it has been thought that proteins recycling to SSVs and newly synthesized proteins reaching SLMVs do so after trafficking through endosomal intermediates. More recently, it has been shown (Takei et al., 1996; Murthy and Stevens, 1998) that in primary neurons, SSVs can reform directly from the plasma membrane via clathrin-coated pits. Moreover, work from Huttner’s group (Schmidt et al., 1997) indicates that SLMVs can bud from the plasma membrane in PC12 cells. This is supported by data from the Kelly laboratory demonstrating that there are two routes to the SLMV from the plasma membrane in PC12 cells. One pathway is AP3 dependent and utilizes an endosomal intermediate (Faundez et al., 1997, 1998; Lichtenstein et al., 1998). The second route is clathrin and dynamin dependent and involves direct budding from the plasma membrane (Schmidt et al., 1997; Shi 1998). There is a growing body of data in primary adrenergic and neuronal cells of a differential secretagogue responsiveness of RSO subpopulations (Kuromi and Kidokoro, 1998; Smith et al., 1998). The two different routes may be an extension of this differential responsiveness. Our data strongly suggest that P-selectin passes from the plasma membrane to the SLMV via an endosomal intermediate.
Why would a DCG-derived protein pass to SLMVs via the more complex of the two possible routes? Passage through a sorting compartment might be needed to effectively separate proteins that are only to be found in DCGs and therefore are en route back to the forming DCGs in the TGN from those that can transfer. The endosome is primarily a sorting compartment.
There is an extensive literature examining the poststimulation morphology of neurons and endocrine cells. Several groups have observed an increase in number of small vesicles, potentially SLMVs, relative to DCGs after stimulation (Palay and Palade, 1955; Geffen and Ostenberg, 1969; Smith, 1971; Thureson-Klein, 1983; Patzak and Winkler, 1986). We have now examined the behavior of individual proteins within this context. Our data suggest that at least some of these small vesicles could be functionally active regulated secretory organelles.
Recycling of DCG membranes after stimulation from primary chromaffin cells has also been studied (Patzak et al., 1984; Patzak and Winkler, 1986; Hunter and Phillips, 1989; von Grafenstein and Knight, 1992, 1993; von Grafenstein et al., 1992; Hurtley, 1993). von Grafenstein and Knight have demonstrated that after stimulation the DCG protein DBH colocalizes with an internalized fluid phase tracer in an uncharacterized compartment distinct from DCGs that is responsive to secretagogue stimulation (von Grafenstein and Knight, 1992). The generation of this secretagogue-responsive endosome is blocked by low temperature (von Grafenstein and Knight, 1993). This compartment may be closely related to the rab5-positive endosome that contains both synaptophysin and DBH after stimulation in primary noradrenergic neurons (Partoens et al., 1998). The relationship between these studies and our own findings has not yet been clarified.
Our data support the hypothesis of Huttner and colleagues (Bauerfeind et al., 1995), who characterized catecholamine-containing small vesicles in rat vas deferens that appear to be a hybrid of the SSV and the DCG. They suggested that these resulted from the mixing of the two membrane populations after exocytosis. Our data provide evidence of the trafficking route that would, in principle, be required for this to happen.
Another important consequence of our findings relates to the fact that in cells with both DCGs and SLMVs, differential release of the two kinds of transmitter; peptides, and small molecules has been demonstrated (Lundberg et al., 1986; Iverfeldt et al., 1989; Verhage et al., 1991; Franck et al., 1993). This differential release depends on the two organelles having different content and differential responsiveness to stimulation. Both of these will presumably depend on their membrane composition and therefore on the efficient sorting of RSO membrane proteins in the cell. Recycling must make an even more significant contribution to the maintenance of RSOs in tissues (given their low levels of synthesis) compared with culture-adapted cell lines that are actively dividing and synthesizing new membrane proteins. This suggests that in vivo the postexocytic trafficking and, in particular, the ability of the cell to separate the two pools of membrane proteins from each other is of vital importance. In light of this it is surprising that VAMP and synaptotagmin, which are part of the docking and fusion machinery (Ferro-Novick and Jahn, 1994; Schiavo et al., 1995), are able to move into the SLMV. This would indicate that the amount of these proteins in the SLMV is not tightly controlled. Alternatively, the number of SLMV could increase after stimulation such that the concentration of these proteins per vesicle would remain unchanged. However, the level of synaptophysin in the SLMV peak does not rise after stimulation, suggesting that the numbers of vesicles is relatively unchanged. Moreover, others have demonstrated that a 50-fold change in the ratio of VAMP to synaptophysin is possible in the SLMVs of PC12 cells (Grote et al., 1995), suggesting that SLMVs do not tightly regulate the concentration of these membrane proteins. One intriguing alternate possibility is that the cell does allow the number of SLMVs to rise but retains a tight control on the total amount of synaptophysin permitted to enter a regulated secretory compartment, for some reason related to its as-yet-unidentified function. Clearly, secretagogue action has profound effects on the intracellular distribution of these membrane proteins, and further studies will be needed to unravel all of the implications of these findings.
Finally, although we have shown transfer in one direction, from the DCG to the SLMV, we predict that the converse will also happen, albeit with much slower kinetics. It may be that the presence of a lysosomal targeting signal in P-selectin (Green et al., 1994; Blagoveshchenskaya et al., 1998a,b, 1999; Straley et al., 1998) reduces this component of its trafficking. We are currently carrying out a detailed characterization of the targeting signals in P-selectin and, should it prove possible to reduce lysosomal targeting without affecting RSO targeting, we will be able to examine in detail both recycling to the granule and transfer from the SLMV to the granule after stimulation.
ACKNOWLEDGMENTS
We are grateful to John Norcott for contributing his expertise to this project. We also acknowledge the contribution of Dr. Hiroshi Nomoto (Gifu Pharmaceutical University, Japan) in constructing the TfnR chimera. We thank the anonymous referee who suggested the experiment shown in Figure 7. The GFP-VAMP construct was the generous gift of Roberto Solari and Nicki Thompson (Glaxo-Wellcome, Stevenage, UK). We thank Clare Futter for reading the manuscript. The anti-GFP antiserum was a kind gift of David T. Shima (ICRF, London), and the anti-synaptotagmin was a kind gift from Gary E. Dean, (Cincinnati, OH). This work was funded by a Wellcome Trust project grant to D.F.C. supporting J.E.S. and M.A., a Wellcome Trust fellowship to A.D.B., and a Medical Research Council program grant to D.F.C.
Abbreviations used:
- DBH
dopamine β-hydroxylase
- DCG
dense core granule
- GFP
green fluorescent protein
- RSO
regulated secretory organelle
- SLMV
synaptic-like microvesicle
- SSV
small synaptic vesicle
- Tfn
transferrin
- TfnR
transferrin receptor
- TGN
trans-Golgi network
- VAMP
vesicle-associated membrane protein
REFERENCES
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauerfeind R, Jelinek R, Hellwig A, Huttner WB. Neurosecretory vesicles can be hybrids of synaptic vesicles and secretory granules. Proc Natl Acad Sci USA. 1995;92:7342–7346. doi: 10.1073/pnas.92.16.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A balance of opposing signals within the cytoplasmic tail controls the lysosomal targeting of P-selectin. J Biol Chem. 1998a;273:27896–27903. doi: 10.1074/jbc.273.43.27896. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt E W, Culter DF. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J Cell Biol. 1999;145:1419–1433. doi: 10.1083/jcb.145.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998b;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Cameron PL, Südhof TC, Jahn R, De Camilli P. Colocalization of synaptophysin with transferrin receptors: implications for synaptic vesicle biogenesis. J Cell Biol. 1991;115:151–164. doi: 10.1083/jcb.115.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clift-O’Grady L, Linstedt AD, Lowe AW, Grote E, Kelly RB. Biogenesis of synaptic vesicle-like structures in a pheochromocytoma cell line PC-12. J Cell Biol. 1990;110:1693–1703. doi: 10.1083/jcb.110.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LP, Cutler DF. Sorting between secretory pathways. In: Magee A, Wileman T, editors. Protein Traffic: A Practical Approach. Oxford, UK: Oxford University Press; 1992. pp. 59–85. [Google Scholar]
- Cutler DF, Cramer LP. Sorting during transport to the surface of PC12 cells: divergence of synaptic vesicle and secretory granule proteins. J Cell Biol. 1990;110:721–730. doi: 10.1083/jcb.110.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnos C, Clift-O’Grady L, Kelly RB. Biogenesis of synaptic vesicles in vitro. J Cell Biol. 1995;130:1041–1049. doi: 10.1083/jcb.130.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elferink LA, Peterson MR, Scheller RH. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. ADP ribosylation factor1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol. 1997;138:505–512. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G, Stahl B, Walch C, Solimena C, Takei K, Daniels L, Khoklatchev A, De Camilli P, Südhof TC, Jahn R. Localization of rab5 to synaptic vesicles identifies an endosomal intermediate in synaptic vesicle recycling pathway. Eur J Cell Biol. 1994;65:319–326. [PubMed] [Google Scholar]
- Fleming JC, Berger G, Guichard J, Cramer EM, Wagner DD. The transmembrane domain enhances granular targeting of P-selectin. Eur J Cell Biol. 1998;75:331–343. doi: 10.1016/s0171-9335(98)80066-6. [DOI] [PubMed] [Google Scholar]
- Franck J, Brodin E, Fried G. Differential release of endogenous 5-hydroxytryptamine, substance P, and neurokinin A from rat ventral spinal cord in response to electrical stimulation. J Neurochem. 1993;61:704–711. doi: 10.1111/j.1471-4159.1993.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Futter CE, Pearse A, Hewlett LJ, Hopkins CR. Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J Cell Biol. 1996;132:1011–1023. doi: 10.1083/jcb.132.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffen LB, Ostenberg A. Distribution of granular vesicles in normal and constricted sympathetic neurons. J Physiol. 1969;204:583–594. doi: 10.1113/jphysiol.1969.sp008933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Setiadi H, McEver RP, Kelly RB. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Hao J, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- Grote E, Kelly RB. Endocytosis of VAMP is facilitated by a synaptic vesicle targeting signal. J Cell Biol. 1996;132:537–547. doi: 10.1083/jcb.132.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Hunter A, Phillips JH. The recycling of a secretory granule membrane protein. Exp Cell Res. 1989;182:445–460. doi: 10.1016/0014-4827(89)90249-8. [DOI] [PubMed] [Google Scholar]
- Hurtley SM. Recycling of a secretory granule membrane protein after stimulated secretion. J Cell Sci. 1993;106:649–656. doi: 10.1242/jcs.106.2.649. [DOI] [PubMed] [Google Scholar]
- Iverfeldt K, Serfozo P, Diaz-Arnesto L, Bartfai T. Differential release of coexisting neurotransmitters: frequency dependence of the efflux of substance P, thyrotropin releasing hormone and [3H]serotonin from tissue slices of rat ventral spinal cord. Acta Physiol Scand. 1989;137:63–71. doi: 10.1111/j.1748-1716.1989.tb08721.x. [DOI] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lah JJ, Burry RW. Synaptophysin has a selective distribution in early endosomes of PC12 cells. J Neurocytol. 1993;22:92–101. doi: 10.1007/BF01181573. [DOI] [PubMed] [Google Scholar]
- Lichtenstein Y, Desnos C, Faundez V, Kelly RB, Clift-O’Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci USA. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AW, Madeddu L, Kelly RB. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988;106:51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg JM, Rudehill A, Sollevi A, Theodorsson-Norheim E, Hamberger B. Frequency- and reserpine-dependent chemical coding of sympathetic transmission: differential release of noradrenaline and neuropeptide Y from pig spleen. Neurosci Lett. 1986;63:96–100. doi: 10.1016/0304-3940(86)90020-0. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Stevens CF. Synaptic vesicles retain their identity through the endocytic cycle. Nature. 1998;392:497–501. doi: 10.1038/33152. [DOI] [PubMed] [Google Scholar]
- Norcott JP, Solari R, Cutler DF. Targetting of P-selectin to two regulated secretory organelles in PC12 cells. J Cell Biol. 1996;134:1229–1240. doi: 10.1083/jcb.134.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay SL, Palade GE. The fine structure of neurons. J Biophys Biochem Cytol. 1955;1:69–88. doi: 10.1083/jcb.1.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papini E, Rosetto O, Cutler DF. VAMP-2 is associated with dense-core secretory granules in PC12 neuroendocrine cells. J Biol Chem. 1995;270:1332–1336. doi: 10.1074/jbc.270.3.1332. [DOI] [PubMed] [Google Scholar]
- Partoens P, Slembrouck D, Quatacker J, Baudhuin P, Courtoy PJ, De Potter WP. Retrieved constituents of large dense-cored vesicles and synaptic vesicles intermix in stimulation-induced early endosomes of noradrenergic neurons. J Cell Sci. 1998;111:681–689. doi: 10.1242/jcs.111.6.681. [DOI] [PubMed] [Google Scholar]
- Patzak A, Bock G, Gischer-Colbrie R, Schauenstein K, Schmidt W, Lingg G, Winkler H. Exocytotic exposure and retrieval of membrane antigens of chromaffin granules: quantitative evaluation of immunofluorescence on the surface of chromaffin cells. J Cell Biol. 1984;98:1817–1824. doi: 10.1083/jcb.98.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzak A, Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after immunolabelling. J Cell Biol. 1986;102:510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JH. Chromaffin granule biogenesis and the exocytosis/endocytosis cycle. In: Rosenheck, Lelkes, editors. Stimulus-Secretion Coupling in Chromaffin Cells. Vol. 1. Boca Raton, FL: CRC Press; 1987. pp. 31–54. [Google Scholar]
- Régnier-Vigouroux A, Tooze SA, Huttner WB. Newly synthesized synaptophysin is transported to the synaptic-like microvesicle via constitutive secretory vesicles and the plasma membrane. EMBO J. 1991;10:3589–3601. doi: 10.1002/j.1460-2075.1991.tb04925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm H, Wiedenmann B, Betz H. Molecular characterization of synaptophysin, a major calcium-binding protein of the synaptic vesicle membrane. EMBO J. 1986;5:535–541. doi: 10.1002/j.1460-2075.1986.tb04243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman NH, Maxfield F R. Fusion accessibility of endocytic compartments along the recycling and lysosomal endocytic pathways in intact cells. J Cell Biol. 1989;109:2097–2104. doi: 10.1083/jcb.109.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo G, Gmachl MJ, Stenbeck G, Sollner TH, Rothman JE. A possible docking and fusion particle for synaptic transmission. Nature. 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer ES, Kelly RB. Selective packaging of human growth hormone into synaptic vesicles in a rat neuronal (PC12) cell line. J Cell Biol. 1985;101:667–676. doi: 10.1083/jcb.101.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Faundez V, Roos J, Dell’Angelica EC, Kelly RB. Neuroendocrine synaptic vesicles are formed in vitro by both clathrin-dependent and clathrin-independent pathways. J Cell Biol. 1998;143:947–955. doi: 10.1083/jcb.143.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD. Summing up: some implications of the neuron as a secreting cell. Philos Trans R Soc Lond Ser B Biol Sci. 1971;261:423–437. doi: 10.1098/rstb.1971.0076. [DOI] [PubMed] [Google Scholar]
- Smith C, Moser T, Xu T, Neher E. Cytosolic Ca2+ acts by two separate pathways to modulate the supply of release-competent vesicles in chromaffin cells. Neuron. 1998;20:1243–1253. doi: 10.1016/s0896-6273(00)80504-8. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley KS, Daugherty BL, Aeder SE, Hockenson AL, Kim K, Green SA. An atypical sorting determinant in the cytoplasmic domain of P-selectin mediates endosomal sorting. Mol Biol Cell. 1998;9:1683–1694. doi: 10.1091/mbc.9.7.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. They synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thureson-Klein A. Exocytosis from large and small dense cored vesicles in noradrenergic nerve terminals. Neuroscience. 1983;10:245–259. doi: 10.1016/0306-4522(83)90132-x. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Stinchcombe JC. Biogenesis of secretory granules. Semin Cell Biol. 1992;3:357–366. doi: 10.1016/1043-4682(92)90021-m. [DOI] [PubMed] [Google Scholar]
- Verhage M, McMahon HT, Ghijsen WE, Boomsma F, Scholten G, Wiegant VM, Nicholls DG. Differential release of amino acids, neuropeptides, and catecholamines from isolated nerve terminals. Neuron. 1991;6:517–524. doi: 10.1016/0896-6273(91)90054-4. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H, Borges R, Knight DE. The effect of botulinum toxin type D on triggered and constitutive exocytosis/endocytosis cycles in cultures of bovine adrenal medullary cells. FEBS Lett. 1992;298:118–122. doi: 10.1016/0014-5793(92)80035-f. [DOI] [PubMed] [Google Scholar]
- von Grafenstein H, Knight DE. Membrane recapture and early triggered secretion from the newly formed endocytic compartment in bovine chromaffin cells. J Physiol. 1992;453:15–31. doi: 10.1113/jphysiol.1992.sp019215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Grafenstein H, Knight DE. Triggered exocytosis and endocytosis have different requirements for calcium and nucleotides in permeablized bovine chromaffin cells. J Membr Biol. 1993;134:1–13. doi: 10.1007/BF00233471. [DOI] [PubMed] [Google Scholar]
- Walch-Solimena C, Takei K, Marek KL, Midyett K, Südhof TC, De Camilli P, Jahn R. Synaptotagmin: a membrane constituent of neuropeptide-containing large dense-core vesicles. J Neurosci. 1993;13:3895–3903. doi: 10.1523/JNEUROSCI.13-09-03895.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Whalley T, Terasaki M, Cho M-S, Vogel SS. Direct membrane retrieval into large vesicles after exocytosis in sea urchin eggs. J Cell Biol. 1995;131:1183–1192. doi: 10.1083/jcb.131.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]