Abstract
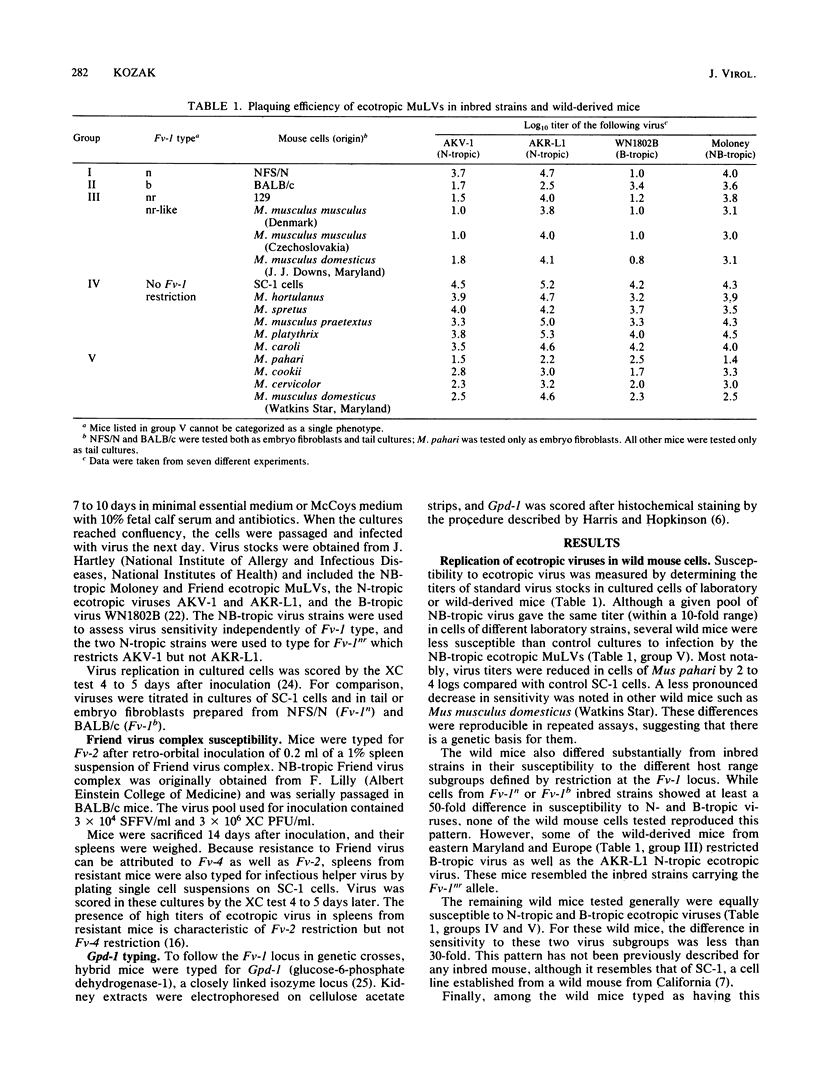
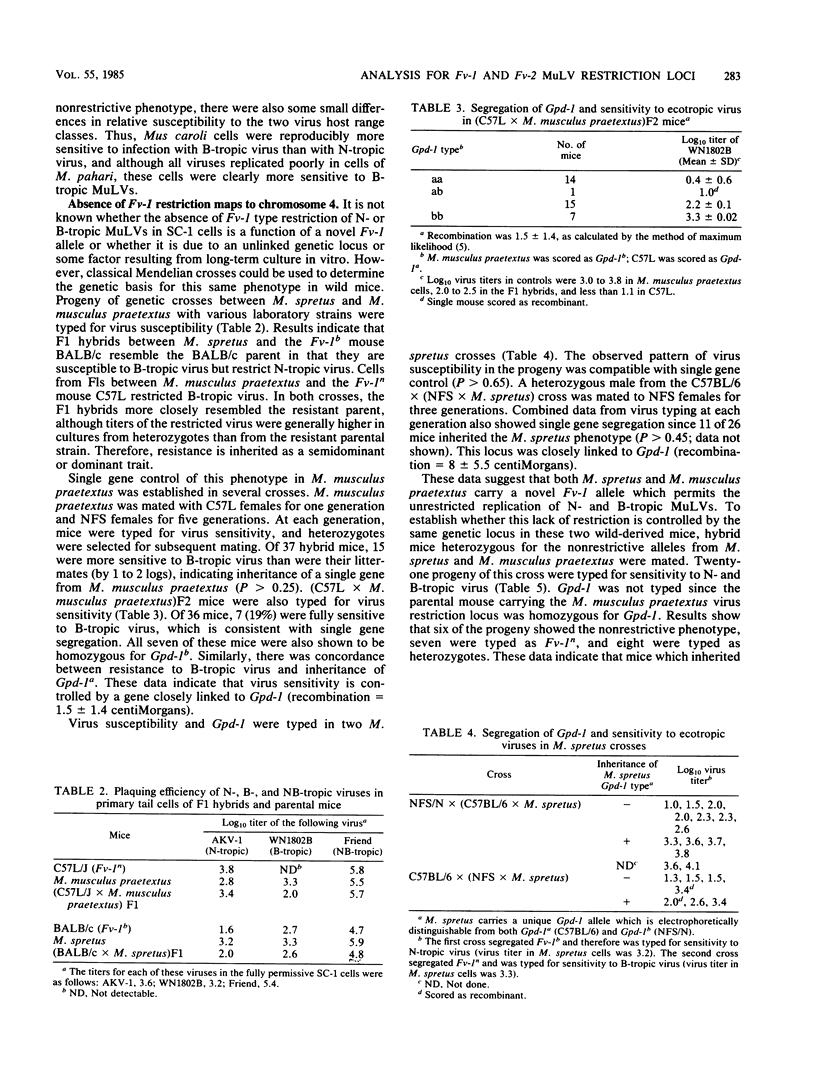
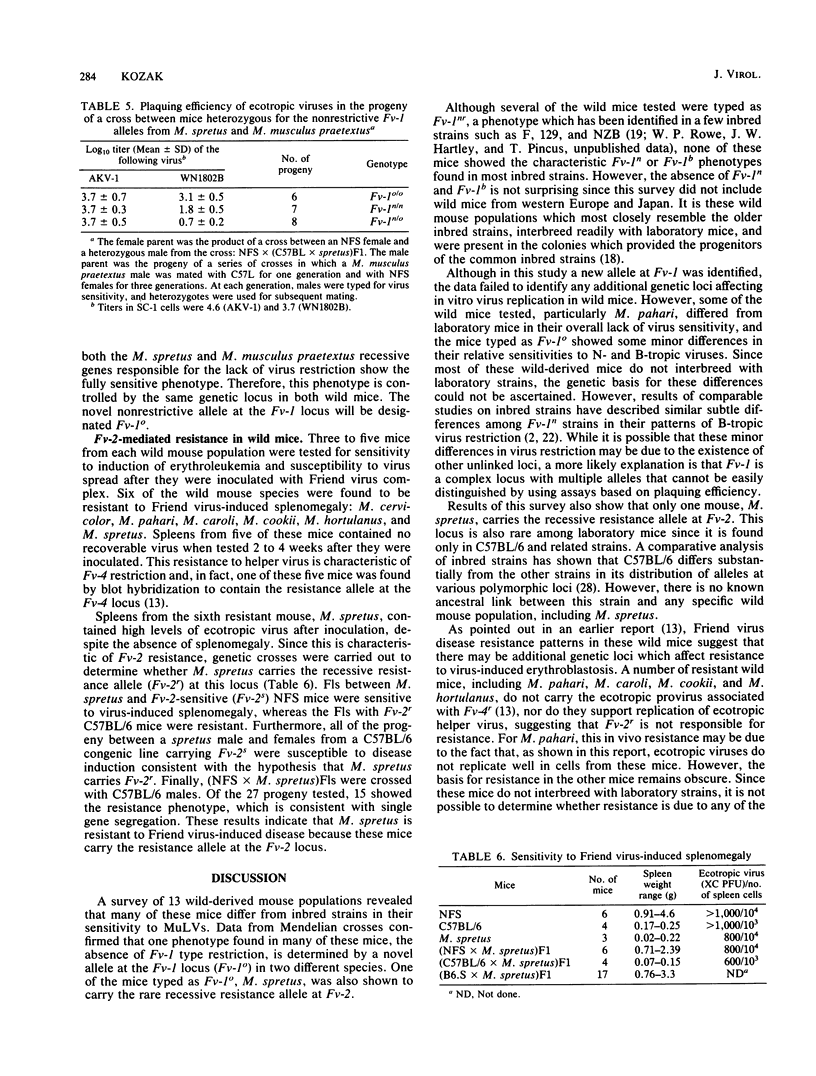
Wild-derived mice originally obtained from Asia, Africa, North America, and Europe were typed for in vitro sensitivity to ecotropic murine leukemia viruses and for susceptibility to Friend virus-induced disease. Cell cultures established from some wild mouse populations were generally less sensitive to exogenous virus than were cell cultures from laboratory mice. Wild mice also differed from inbred strains in their in vitro sensitivity to the host range subgroups defined by restriction at the Fv-1 locus. None of the wild mice showed the Fv-1n or Fv-1b restriction patterns characteristic of most inbred strains, several mice resembled the few inbred strains carrying Fv-1nr, and most differed from laboratory mice in that they did not restrict either N- or B-tropic murine leukemia viruses. Analysis of genetic crosses of Mus spretus and Mus musculus praetextus demonstrated that the nonrestrictive phenotype is controlled by a novel allele at the Fv-1 locus, designated Fv-10. The wild mice were also tested for sensitivity to Friend virus complex-induced erythroblastosis to type for Fv-2. Only M. spretus was resistant to virus-induced splenomegaly and did not restrict replication of Friend virus helper murine leukemia virus. Genetic studies confirmed that this mouse carries the resistance allele at Fv-2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. A., Croizat H., Eskinazi D. A washable macromolecule from Fv2rr marrow negatively regulates DNA synthesis in erythropoietic progenitor cells BFU-E. Cell. 1981 Oct;26(2 Pt 2):233–244. doi: 10.1016/0092-8674(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Benjers B. M., Bassin R. H., Rein A., Gerwin B. I., Duran-Troise G. Mechanism of restriction of murine leukemia viruses varies between different strains of Fv-1n mice. Int J Cancer. 1979 Nov 15;24(5):600–607. doi: 10.1002/ijc.2910240513. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Jensen F. C., Lerner R. A. In vitro construction of a B-tropic virus by recombination: B-tropism is a cryptic phenotype of xenotropic murine retroviruses. Proc Natl Acad Sci U S A. 1980 May;77(5):2989–2993. doi: 10.1073/pnas.77.5.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Besmer P., Chu L., Baltimore D. Growth of pseudotypes of vesicular stomatitis virus with N-tropic murine leukemia virus coats in cells resistant to N-tropic viruses. J Virol. 1973 Sep;12(3):659–662. doi: 10.1128/jvi.12.3.659-662.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Odaka T. Cellular expression of murine leukemia virus gp70-related antigen on thymocytes of uninfected mice correlates with Fv-4 gene-controlled resistance to Friend leukemia virus infection. Virology. 1983 Jul 15;128(1):127–139. doi: 10.1016/0042-6822(83)90324-0. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A., Gromet N. J., Ikeda H., Buckler C. E. A unique sequence related to the ecotropic murine leukemia virus is associated with the Fv-4 resistance gene. Proc Natl Acad Sci U S A. 1984 Feb;81(3):834–837. doi: 10.1073/pnas.81.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krontiris T. G., Soeiro R., Fields B. N. Host restriction of Friend leukemia virus. Role of the viral outer coat. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2549–2553. doi: 10.1073/pnas.70.9.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Moll B., Rowe W. P. A procedure for culture of cells from mouse tail biopsies: brief communication. J Natl Cancer Inst. 1978 Feb;60(2):477–478. [PubMed] [Google Scholar]
- Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970 Jul;45(1):163–169. [PubMed] [Google Scholar]
- Mak T. W., Axelrad A. A., Bernstein A. Fv-2 locus controls expression of Friend spleen focus-forming virus-specific sequences in normal and infected mice. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5809–5812. doi: 10.1073/pnas.76.11.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Kozak C. A., Yetter R. A., Hartley J. W. Unique features of retrovirus expression in F/St mice. J Virol. 1982 Jul;43(1):1–7. doi: 10.1128/jvi.43.1.1-7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odaka T., Ikeda H., Yoshikura H., Moriwaki K., Suzuki S. Fv-4: gene controlling resistance to NB-tropic Friend murine leukemia virus. Distribution in wild mice, introduction into genetic background of BALB/c mice, and mapping of chromosomes. J Natl Cancer Inst. 1981 Nov;67(5):1123–1127. [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. I. Tissue culture studies of naturally occurring viruses. J Exp Med. 1971 Jun 1;133(6):1219–1233. doi: 10.1084/jem.133.6.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincus T., Hartley J. W., Rowe W. P. A major genetic locus affecting resistance to infection with murine leukemia viruses. IV. Dose-response relationships in Fv-1-sensitive and resistant cell cultures. Virology. 1975 Jun;65(2):333–342. doi: 10.1016/0042-6822(75)90039-2. [DOI] [PubMed] [Google Scholar]
- Risser R. Friend erythroleukemia antigen. A viral antigen specified by spleen focus-forming virus and differentiation antigen controlled by the Fv-2 locus. J Exp Med. 1979 May 1;149(5):1152–1167. doi: 10.1084/jem.149.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Sato H. Genetic mapping of the Fv-1 lcous of the mouse. Science. 1973 May 11;180(4086):640–641. doi: 10.1126/science.180.4086.640. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Bubbers J. E., Plata F., Lilly F. Origin of spleen colonies generated by Friend virus-infected cells in mice. Cancer Res. 1978 Sep;38(9):2729–2733. [PubMed] [Google Scholar]
- Suzuki S. FV-4: a new gene affecting the splenomegaly induction by Friend leukemia virus. Jpn J Exp Med. 1975 Dec;45(6):473–478. [PubMed] [Google Scholar]
- Taylor B. A. Genetic relationships between inbred strains of mice. J Hered. 1972 Mar-Apr;63(2):83–86. doi: 10.1093/oxfordjournals.jhered.a108235. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Kiggans J. O., Yang D. M., Ou C. Y., Tennant R. W., Brown A., Bassin R. H. Synthesis and circularization of N- and B-tropic retroviral DNA Fv-1 permissive and restrictive mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2994–2998. doi: 10.1073/pnas.77.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikura H., Odaka T. Surface antigen expressed in hematopoietic cells derived from Fv-4r mouse strains. J Natl Cancer Inst. 1982 Jun;68(6):1005–1009. [PubMed] [Google Scholar]


