Abstract
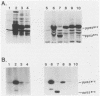
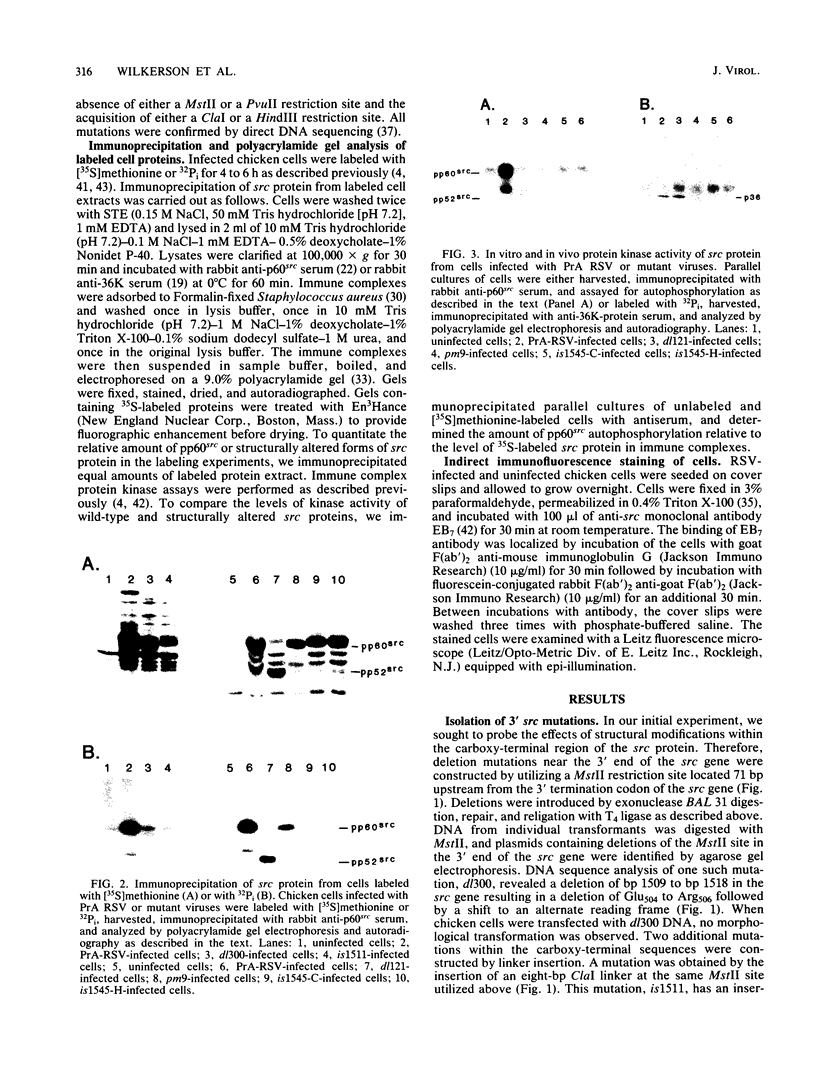
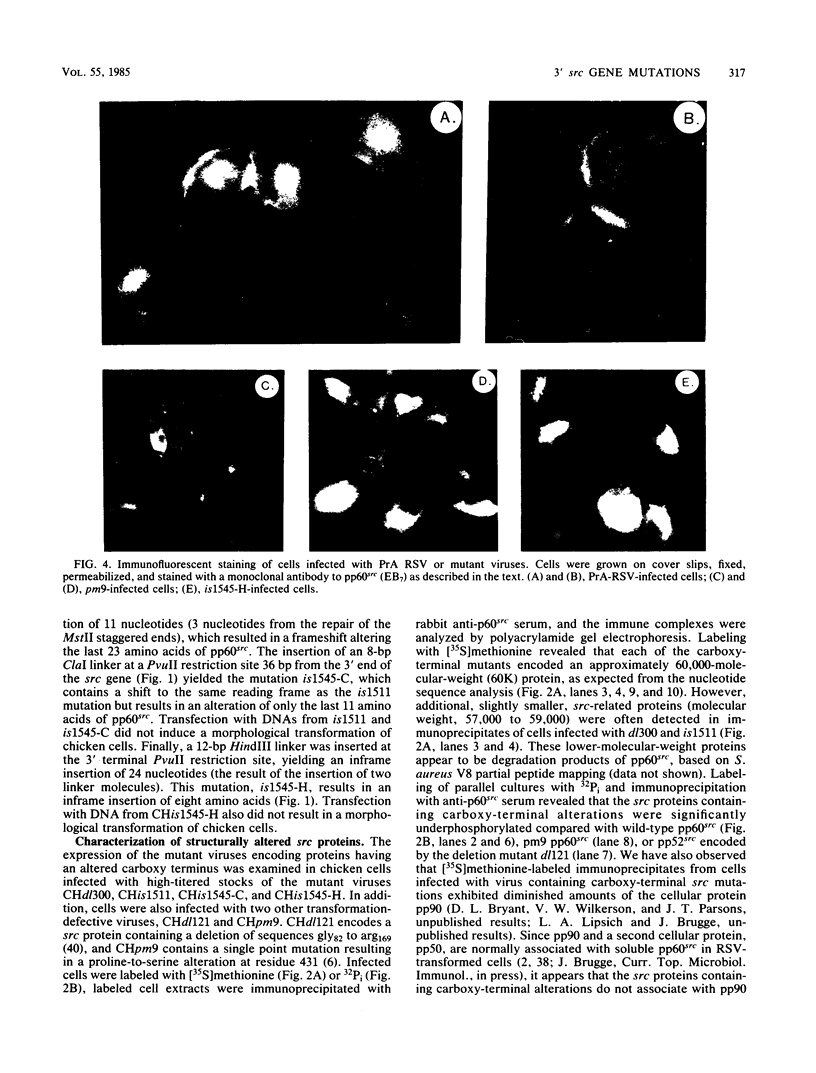
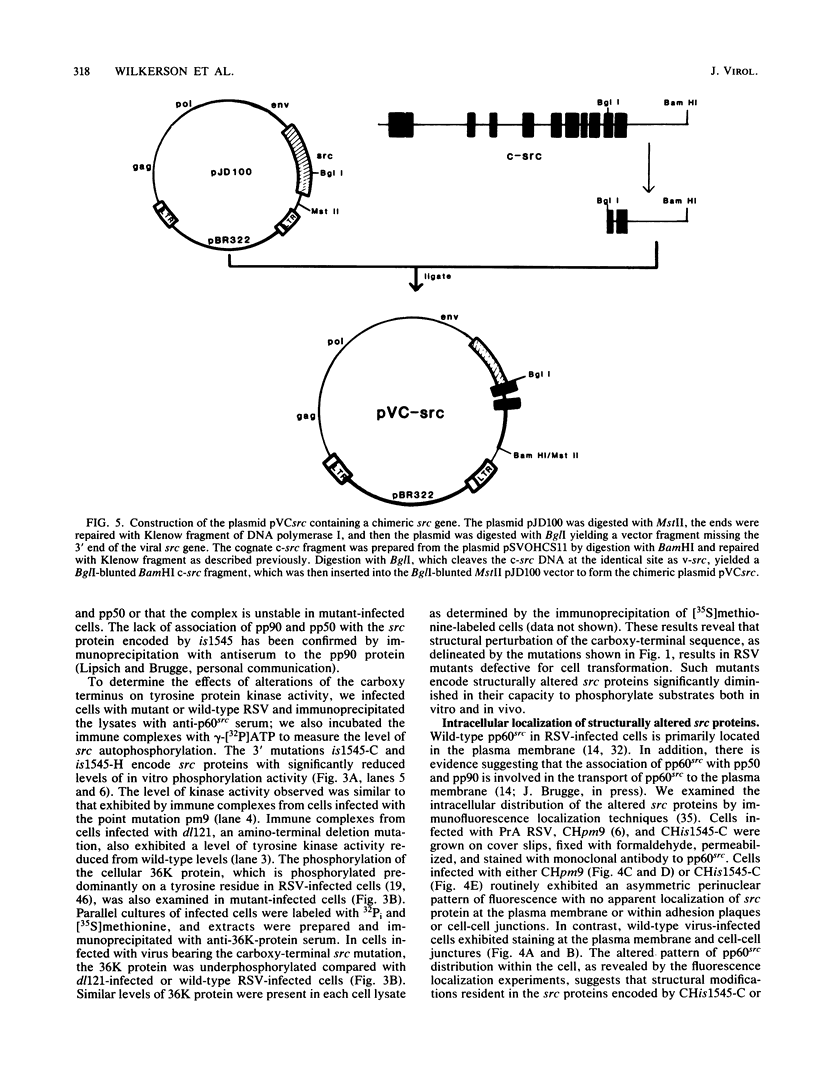
The src gene of Rous sarcoma virus (v-src) and its cellular homolog, the c-src gene, share extensive sequence homology. The most notable differences between these genes reside in the region encoding the carboxy terminus of the src proteins. We constructed mutations within the 3' end of the v-src gene to determine the significance of this region to the transforming potential of the v-src protein, pp60v-src. The mutants CHdl300 and CHis1511 contain mutations that alter the last 23 amino acids of pp60v-src, whereas the mutant CHis1545-C contains a linker insertion that alters the last 11 amino acids of pp60v-src, and the mutant CHis1545-H contains a linker insertion that results in a 9-amino-acid insertion at position 415. Plasmids bearing each of these mutations were unable to transform chicken cells when introduced into these cells by DNA transfection. In addition, the structurally altered src proteins encoded by the mutants had much-reduced levels of tyrosine protein kinase activity in vivo, as measured by autophosphorylation and phosphorylation of the 34,000-Mr cellular protein, and in vitro, as determined by measuring the level of pp60src autophosphorylation. These data indicate that the carboxy-terminal amino acid sequences play an important role in maintaining the structure of the catalytic domain of pp60v-src. In contrast, the transfection of chicken cells with plasmid DNA containing a chimeric v-c-src gene resulted in morphological cell transformation and the synthesis of an enzymatically active hybrid protein. Therefore, the carboxy-terminal sequence alterations observed in the c-src protein do not alone serve to alter the functional activity of a hybrid v-c-src protein appreciably.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Bryant D. L., Parsons J. T. Amino acid alterations within a highly conserved region of the Rous sarcoma virus src gene product pp60src inactivate tyrosine protein kinase activity. Mol Cell Biol. 1984 May;4(5):862–866. doi: 10.1128/mcb.4.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D., Parsons J. T. Site-directed mutagenesis of the src gene of Rous sarcoma virus: construction and characterization of a deletion mutant temperature sensitive for transformation. J Virol. 1982 Nov;44(2):683–691. doi: 10.1128/jvi.44.2.683-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D., Parsons J. T. Site-directed point mutation in the src gene oF rous sarcoma virus results in an inactive src gene product. J Virol. 1983 Mar;45(3):1211–1216. doi: 10.1128/jvi.45.3.1211-1216.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Sefton B. M. Myristic acid, a rare fatty acid, is the lipid attached to the transforming protein of Rous sarcoma virus and its cellular homolog. J Virol. 1985 Jan;53(1):7–12. doi: 10.1128/jvi.53.1.7-12.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson E., Erikson R. L. Structural analysis of the avian sarcoma virus transforming protein: sites of phosphorylation. J Virol. 1979 Feb;29(2):770–781. doi: 10.1128/jvi.29.2.770-781.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Reiss N. A., Schwartz R. J., Hunter T. Three glycolytic enzymes are phosphorylated at tyrosine in cells transformed by Rous sarcoma virus. Nature. 1983 Mar 17;302(5905):218–223. doi: 10.1038/302218a0. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Levinson A. D., Bishop J. M. The protein encoded by the transforming gene of avian sarcoma virus (pp60src) and a homologous protein in normal cells (pp60proto-src) are associated with the plasma membrane. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3783–3787. doi: 10.1073/pnas.77.7.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gilmer T. M., Erikson R. L. Rous sarcoma virus transforming protein, p60src, expressed in E. coli, functions as a protein kinase. Nature. 1981 Dec 24;294(5843):771–773. doi: 10.1038/294771a0. [DOI] [PubMed] [Google Scholar]
- Gilmer T. M., Parsons J. T. Analysis of cellular integration sites in avian sarcoma virus infected duck embryo cells. J Virol. 1979 Dec;32(3):762–769. doi: 10.1128/jvi.32.3.762-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild B. C., Erikson R. L., Strominger J. L. HLA-A2 and HLA-B7 antigens are phosphorylated in vitro by rous sarcoma virus kinase (pp60v-src) at a tyrosine residue encoded in a highly conserved exon of the intracellular domain. Proc Natl Acad Sci U S A. 1983 May;80(10):2894–2898. doi: 10.1073/pnas.80.10.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield P. E., Rafield L. F., Gilmer T. M., Parsons J. T. Molecular cloning of avian sarcoma virus closed circular DNA: structural and biological characterization of three recombinant clones. J Virol. 1980 Oct;36(1):271–279. doi: 10.1128/jvi.36.1.271-279.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Takeya T., Cross F. R., Hanafusa T., Hanafusa H. Rous sarcoma virus variants that carry the cellular src gene instead of the viral src gene cannot transform chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4424–4428. doi: 10.1073/pnas.81.14.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Krueger J. G., Wang E., Goldberg A. R. Evidence that the src gene product of Rous sarcoma virus is membrane associated. Virology. 1980 Feb;101(1):25–40. doi: 10.1016/0042-6822(80)90480-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Little C. D., Chen W. T. Masking of extracellular collagen and the co-distribution of collagen and fibronectin during matrix formation by cultured embryonic fibroblasts. J Cell Sci. 1982 Jun;55:35–50. doi: 10.1242/jcs.55.1.35. [DOI] [PubMed] [Google Scholar]
- Macara I. G., Marinetti G. V., Balduzzi P. C. Transforming protein of avian sarcoma virus UR2 is associated with phosphatidylinositol kinase activity: possible role in tumorigenesis. Proc Natl Acad Sci U S A. 1984 May;81(9):2728–2732. doi: 10.1073/pnas.81.9.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Levintow L., Varmus H. E., Bishop J. M., Kawai S. Two cellular proteins that immunoprecipitate with the transforming protein of Rous sarcoma virus. Virology. 1981 Sep;113(2):736–751. doi: 10.1016/0042-6822(81)90202-6. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Expression of v-src and chicken c-src in rat cells demonstrates qualitative differences between pp60v-src and pp60c-src. Cell. 1984 May;37(1):131–139. doi: 10.1016/0092-8674(84)90308-8. [DOI] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Isolation and partial characterization of a monoclonal antibody to the Rous sarcoma virus transforming protein pp60src. J Virol. 1983 Mar;45(3):1190–1194. doi: 10.1128/jvi.45.3.1190-1194.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., McCarley D. J., Ely C. M., Benjamin D. C., Parsons J. T. Monoclonal antibodies to Rous sarcoma virus pp60src react with enzymatically active cellular pp60src of avian and mammalian origin. J Virol. 1984 Aug;51(2):272–282. doi: 10.1128/jvi.51.2.272-282.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons S. J., Riley S. C., Mullen E. E., Brock E. J., Benjamin D. C., Kuehl W. M., Parsons J. T. Immune response to the src gene product in mice bearing tumors induced by injection of avian sarcoma virus-transformed mouse cells. J Virol. 1979 Oct;32(1):40–46. doi: 10.1128/jvi.32.1.40-46.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Henderson L. E., Oroszlan S., Garber E. A., Hanafusa H. Amino terminal myristylation of the protein kinase p60src, a retroviral transforming protein. Science. 1985 Jan 25;227(4685):427–429. doi: 10.1126/science.3917576. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shalloway D., Coussens P. M., Yaciuk P. Overexpression of the c-src protein does not induce transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7071–7075. doi: 10.1073/pnas.81.22.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y., Whitman M., Cantley L. C., Erikson R. L. Evidence that the Rous sarcoma virus transforming gene product phosphorylates phosphatidylinositol and diacylglycerol. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2117–2121. doi: 10.1073/pnas.81.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]