Abstract
The Schizosaccharomyces pombe win1-1 mutant has a defect in the G2-M transition of the cell cycle. Although the defect is suppressed by wis1+ and wis4+, which are components of a stress-activated MAP kinase pathway that links stress response and cell cycle control, the molecular identity of Win1 has not been known. We show here that win1+ encodes a polypeptide of 1436 residues with an apparent molecular size of 180 kDa and demonstrate that Win1 is a MAP kinase kinase kinase that phosphorylates and activates Wis1. Despite extensive similarities between Win1 and Wis4, the two MAP kinase kinase kinases have distinct functions. Wis4 is able to compensate for loss of Win1 only under unstressed conditions to maintain basal Wis1 activity, but it fails to suppress the osmosignaling defect conferred by win1 mutations. The win1-1 mutation is a spontaneous duplication of 16 nucleotides, which leads to a frameshift and production of a truncated protein lacking the kinase domain. We discuss the cell cycle phenotype of the win1-1 cdc25-22 wee1-50 mutant and its suppression by wis genes.
INTRODUCTION
Investigations on the fission yeast Schizosaccharomyces pombe have been central in unraveling the mechanisms that regulate cell cycle transitions, in particular entry into mitosis from G2. Genetical analysis led to the identification of Cdc2, and of its regulators Cdc25 and Wee1 as key players in G2-M regulation. Further investigations have led to the identification of several other genes whose products play a rate-limiting role in the transition into mitosis (MacNeill and Fantes, 1995).
The S. pombe win1-1 mutant was isolated as a mutation that reverses the effect of wee1-50 in suppressing the temperature-sensitive cell cycle arrest of cdc25-22 (Ogden and Fantes, 1986). Among five wis genes isolated as multicopy suppressors of the cdc phenotype of the triple mutant win1-1 cdc25-22 wee1-50 (Warbrick and Fantes, 1992), wis1+ and wis4+ encode a MAP kinase kinase (MAPKK) and a MAP kinase kinase kinase (MAPKKK), respectively (Warbrick and Fantes, 1991; Samejima et al., 1997). Identification of the win1+ gene is crucial for understanding how these genes affect the cell cycle phenotype of win1-1 cdc25-22 wee1-50. Several attempts to clone the win1+ gene have been made, but its molecular identity remains unknown (Warbrick and Fantes, 1992).
In addition to their cell cycle effects, Wis1 and Wis4 are components of a stress-responsive MAP kinase signaling pathway. Similar pathways exist in a range of eukaryotic cell types (Brewster et al., 1993; Galcheva-Gargova et al., 1994; Han et al., 1994; Millar et al., 1995; Shiozaki and Russell, 1995). In S. pombe, the Wis1 MAPKK is activated in response to osmotic and other types of stress, resulting in increased phosphorylation of the MAP kinase homologue Spc1 (= Sty1 = Phh1) (Millar et al., 1995; Shiozaki and Russell, 1995; Kato et al., 1996). wis1+ and spc1+ are essential for survival under conditions of extreme heat, osmolarity, oxidation, or poor nutrition. Wis4 (= Wik1 = Wak1) is a MAPKKK that phosphorylates and activates Wis1; however, the phenotype of wis4Δ mutants is not as severe as those of wis1 deletion strains (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997). Activation of Wis1 is observed in wis4 deletion strains after exposure to high osmolarity, although activation of Wis1 under these conditions requires phosphorylation of Wis1 at the conserved Ser469 and Thr473 residues (Samejima et al., 1997). These observations suggest that Wis1 is activated by other MAPKKK(s) in response to osmotic stress, and that Wis4 is therefore one of several redundant MAPKKKs that function upstream of Wis1. Genetic evidence suggests that Win1 is also an activator of Wis1, which functions in parallel with Wis4 and plays a major role in osmostress signaling (Samejima et al., 1997).
Here we identify an MAPKKK structurally homologous to Wis4 and present evidence that it is encoded by win1+. We show that Win1 activates Wis1 in vivo and in vitro and is a major transducer of osmostress signaling; cells lacking Win1 function show a reduced basal level of Wis1 activity, and their ability to activate Wis1 in response to osmotic stress is severely compromised. win1-1 is a frameshift mutation, and the mutant gene product is predicted to lack the kinase domain.
MATERIALS AND METHODS
Strains and Media
Standard techniques for handling S. pombe are described elsewhere (Moreno et al., 1991; Alfa et al., 1993). S. pombe cells were grown in YE (yeast extract medium) or EMM2 (minimal medium) (Alfa et al., 1993). All of the strains used for phosphotyrosine assay carried the tagged spc1 allele derived from KS1376 (Shiozaki and Russell, 1995).
PCR Primers
For expression of the catalytic domain of the Win1 protein kinase in S. pombe, the following sequences were used to design PCR primers to clone the last 400 aa of the gene, which was expressed from the pREP1 nmt1 promoter: 5′-GTCGACGTAGTATGGACCAACAA-3′ and 5′-AGATCTAATGGTGATGGTGATGGTGGCGGCCGCCAAGTTCCAACGGAGCACCAT-3′.
Physical Mapping of win1+
One plasmid that contained the tps19+ gene was isolated by complementation of the temperature-sensitive (ts) growth phenotype of the tps19 mutant. A 5-kb BamHI fragment from the plasmid was used to screen a P1 phage library (Hoeheisel et al., 1993) and cosmid libraries (Hoeheisel et al., 1993; Mizukami et al., 1993) by hybridization. For selection of S. pombe transformants carrying P1 phage and cosmids the ura4+ gene and ars1 sequences were first introduced by a method that exploits a bacterial transposon (Morgan et al., 1996). The ura4+–ars1-tagged phage or cosmids were used to transform the win1-1 cdc25-22 wee1-50 mutant to score their ability to suppress the ts phenotype.
Genetic Mapping of win1-1
The win1-1 locus was mapped on chromosome I by mitotic haploidization of the diploid strain win1-1/+ ura1–131/+ lys1–171/+ leu1–32/+ ade6–704/+ mat2–102/h- (Alfa et al., 1993). Preliminary mapping of win1-1 within chromosome I was carried out by random spore analysis in swi5 background (Schmidt et al., 1987), followed by fine mapping by tetrad analysis with markers on the short arm of chromosome I. The relative positions of win1-1, tps19, and rad1-1 were determined by three-point cross-analysis carried out on random spore colonies.
Purification of Win1 Kinases
Win1 proteins tagged with 6× histidine were expressed from the pREP1 nmt1 promoter for 12 h at 30°C in the wis1Δ strain. S. pombe cells were disrupted by glass beads in lysis buffer (50 mM Tris-HCl, pH 8.0, 0.4 M NaCl, 10% glycerol, 10 mM 2-mercaptoethanol, 1 mM PMSF). The cleared lysate was incubated with Ni-NTA-Sepharose beads (Qiagen, Chatsworth, CA) in the presence of 0.1% NP-40 and 20 mM imidazole. The tagged proteins were eluted by the same buffer containing 250 mM imidazole and dialyzed against storage buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 20% glycerol).
Coupled Kinase Assay
Recombinant GST-Wis1(K349R) (0.16 μg) purified from Escherichia coli was incubated in buffer containing 25 μM ATP, with Win1ΔN or Win1ΔN(K1149R) purified from S. pombe equivalent to 2 × 107 cells. After 10 min incubation at 30°C, 1.3 μg of catalytically inactive MAPK substrate was added in the presence of [γ-32P]ATP (0.2 μCi/μl). The mixture was incubated for an additional 10 min at 30°C, and the reactions were stopped by addition of 3× SDS loading buffer. The MAPK substrate GST-Spc1 was purified from an S. pombe wis1Δ strain, essentially according to the method of Shiozaki and Russell (1997). The method for purifying GST-Wis1 from E. coli is described by Samejima et al. (1997).
Phosphotyrosine Assay
S. pombe cells were collected by filtration. Cells were washed once with STOP buffer (50 mM NaF, 100 mM NaCl, 10 mM EDTA, 1 mM NaN3) before being frozen in liquid nitrogen. Spc1 protein was isolated on Ni-NTA beads (Qiagen) in denaturing buffer (6 M guanidine HCl, 0.1 M Na phosphate, 0.1 M Tris-HCl, pH 8.0) as described by Shiozaki and Russell (1995). Spc1 and phosphotyrosine were detected by Western blotting by ECL (Amersham, Buckinghamshire, UK). The primary antibodies used to detect the hemagglutinin (HA) epitope and phosphotyrosine were 12CA5 (Boehringer Mannheim, Indianapolis, IN) and 4G10 (Upstate Biotechnology, Lake Placid, NY), respectively.
RESULTS
Identification of a Candidate Gene for win1+
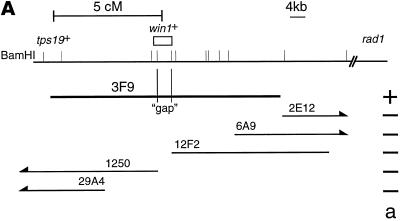
Because attempts to clone the win1+ gene by complementation were unsuccessful (Warbrick and Fantes, 1992), we decided to clone the win1+ gene by positional cloning. win1-1 was mapped on chromosome I, 5 cM from the tps19 locus in the direction of rad1. As a preliminary step to position the win1+ gene on the physical map of the S. pombe genome, the tps19+ gene was cloned and was used as a probe to screen ordered cosmid libraries and a P1 phage library (Hoheisel et al., 1993; Mizukami et al., 1993). 3F9 is one of the positive clones in the P1 phage library, and subsequent analysis revealed that it contains the tps19+ gene at the very end of the insert, which extends in the direction of the rad1 locus (Figure 1A). There is a single region within 3F9 not represented by cosmids from either library. 3F9 suppressed the ts phenotype of the win1-1 cdc25-22 wee1-50 triple mutant, whereas none of the cosmids tested did (Figure 1A), suggesting that the gene responsible for the suppression, or a part of it, lay in the “gap” region. The DNA of the gap region was recovered by PCR amplification, and its DNA sequence was determined. A single ORF spans the gap, suggesting that it is the win1+ gene. The ORF encodes a polypeptide of 1436 amino acid residues with a protein kinase domain at positions 1127–1410 (Figure 1B). The kinase domain of the Win1 protein is most similar to S. pombe Wis4 and Saccharomyces cerevisiae SSK2 and SSK22; within the region, 163 of 291 aa residues (56%) are identical to Wis4. The homology extends beyond the kinase domain throughout the protein, apart from the first ∼180 residues, where we find no homology to any protein in current databases.
Figure 1.
(A) An approximate restriction map of 3F9 and selected cosmids from ordered cosmid libraries (Hoheisel et al., 1993; Mizukami et al., 1993). The positions of BamHI sites, particularly those a long distance apart, may not be accurate. (a) Activity of suppression of the ts phenotype of the win1-1 cdc25-22 wee1-50 mutant. (B) Deduced amino acid sequence of the Win1 MAPKKK. The nucleotide sequence is available from the EMBL, GenBank, and DNA Database of Japan databases under the accession number AJ223190. The amino acid sequences of the products of S. pombe wis4+ and S. cerevisiae SSK2 and SSK22 genes are aligned with the Win1 sequence. Residues conserved among three or four proteins are indicated in white letters on black, and residues conserved between any pair of proteins are indicated in black letters on gray. The first 83 residues of SSK2 are not shown here.
Detection of Full-Length Win1 Protein
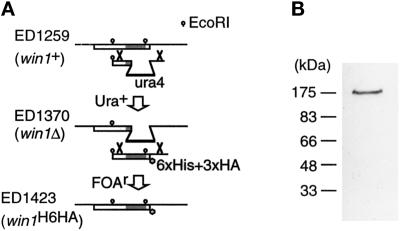
With the aim of identifying the full-size win1+ gene product, we constructed a strain in which the chromosomal win1+ gene was modified such that the Win1 protein was tagged with the HA epitope at the carboxyl terminus (Figure 2A). We confirmed by Southern analysis that the construct had integrated at the intended locus. A polypeptide of 180 kDa was detected by the anti-HA antibody (12CA5) in the cell extract of the tagged strain (Figure 2B), whereas no signal was detected in the cell extract of a parental win1Δ or untagged wild-type strain.
Figure 2.
Detection of the full-length win1+ gene product. (A) Strategy for constructing a strain carrying tagged Win1 (ED1423) by homologous recombination. The win1+ gene in the haploid wild-type strain was disrupted by the S. pombe ura4+ gene to generate a win1Δ strain (ED1370). Then the ura4+ marker of the deletion strain was replaced by a sequence encoding the tagged carboxyl-terminal domain of Win1, using 5-fluoro-orotic acid selection (Grimm et al., 1998). The shaded box indicates the kinase domain. (B) Western blot of S. pombe cell extract from ED1423. Anti-HA antibody (12CA5) was used as the primary antibody.
Win1 Phosphorylates and Activates Wis1 In Vitro
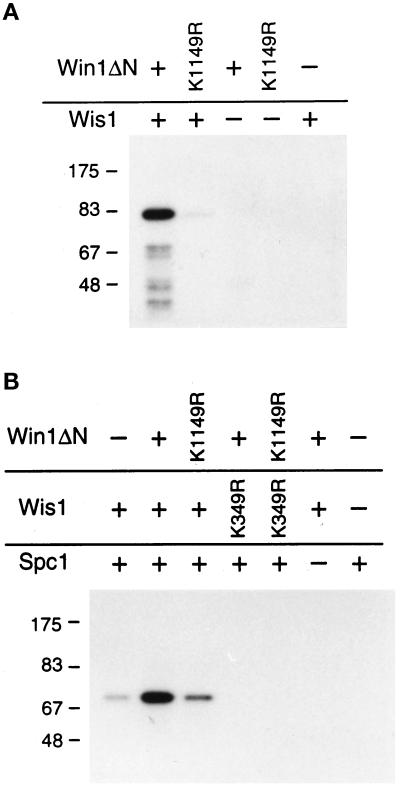
The amino acid sequence similarity of Win1 to MAPKKKs, in particular to Wis4, suggests that Win1 is a protein kinase that phosphorylates Wis1. To test this, we expressed an amino-terminal truncated Win1 protein, Win1ΔN, containing the catalytic domain, in S. pombe. We affinity purified the protein and tested whether it could phosphorylate recombinant GST-Wis1(K349R) in vitro (Figure 3A). This substrate purified from bacteria does not autophosphorylate because of a mutation in the conserved lysine residue essential for ATP binding (Figure 3A, lane 5). The GST-Wis1(K349R) was phosphorylated when incubated with the wild-type Win1ΔN. Incubation with the catalytically defective mutant protein Win1ΔN(K1149R), which has the conserved lysine residue in the ATP binding domain mutated to arginine, had a much reduced effect.
Figure 3.
Win1 phosphorylates and activates Wis1 in vitro. (A) Win1 phosphorylates Wis1. In vitro kinase activity of the catalytic domain of Win1 purified from S. pombe was tested with recombinant GST-Wis1(K349R) as a substrate. (B) Win1 activates Wis1. Wis1 proteins were preincubated with Win1 proteins in the presence of unlabeled ATP. Then, Wis1 kinase activity was tested by assaying incorporation of radioactive phosphate into GST-Spc1 after adding γ-labeled ATP.
We then tested whether this phosphorylation by Win1 led to activation of Wis1 kinase activity. GST-Spc1 was expressed in an S. pombe wis1Δ strain, and the purified protein was used as a substrate to assay Wis1 kinase activity in vitro. GST-Wis1 protein produced in bacteria shows very low kinase activity (Figure 3B, lane 1). Wis1 kinase activity was greatly enhanced after preincubation with Win1 protein in the presence of ATP (Figure 3B, lane 2). The kinase-defective mutant Win1ΔN (K1149R) had a much reduced effect (Figure 3B, lane 3): a likely reason for the difference in Figure 3B between lanes 1 and 3 is a contribution from a minor population of phosphorylated Wis1 molecules (see Figure 3A, lane 2). GST-Wis1(K349R) did not phosphorylate GST-Spc1 under any conditions (Figure 3B, lanes 4 and 5). These observations show that Win1 is able to activate Wis1 in vitro and excludes the possibility that Win1 directly phosphorylates GST-Spc1. These data thus show that phosphorylation by Win1 is sufficient to activate the Wis1 kinase.
Conserved Wis1 Phosphorylation Sites Are Required for Activation by Win1 In Vivo
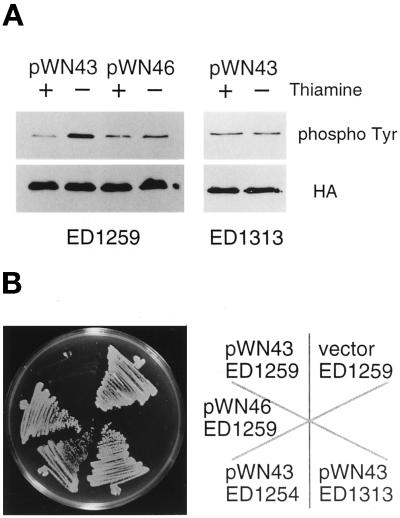
We asked whether Win1 activates Wis1 in vivo by examining the effect of overexpression of the win1+ gene in S. pombe. In many cases, the amino termini of MAPKKK homologues are not essential for their kinase activity, and indeed, the truncated proteins are often more active than their full-length counterparts in vivo (Cairns et al., 1992; Lee and Levin, 1992; Stevenson et al., 1992; Maeda et al., 1995; Samejima et al., 1997; Shiozaki et al., 1997). The carboxyl-terminal 400 residues of Win1, Win1ΔN, which contains the entire catalytic domain, was expressed from the nmt1 promoter, so that its transcription was regulatable by thiamine (Maundrell, 1990). In vivo Wis1 kinase activity was assayed by the level of phosphotyrosine on Spc1 (Shiozaki and Russell, 1995). Overexpression of win1ΔN in wild-type cells resulted in more phosphotyrosine on Spc1, whereas the kinase-defective mutant allele of win1ΔN had no such effect (Figure 4A). We detected no phosphotyrosine in wis1Δ cells under these conditions, consistent with several reports that wis1+ is essential for tyrosine phosphorylation of Spc1 (Millar et al., 1995; Shiozaki et al., 1995).
Figure 4.
Win1 activates Wis1 in vivo. (A) Wis1 kinase activity in vivo was assayed by phosphotyrosine blot of Spc1 protein isolated from the strains indicated. The HA blot shows the relative amounts of HA-tagged Spc1. The truncated win1+ gene was expressed from pREP1 nmt1 promoter by growth of cells in the absence of thiamine for 12 h. (B) The effect of overexpression of active or inactive Win1 catalytic domains was tested in various genetic backgrounds. Each strain was streaked on an EMM plate lacking thiamine, and the win1 gene was overexpressed from the pREP1 nmt1 promoter. The details of the strains given here are ED1259, wild type; ED1313, wis1-4; and ED1254, wis1Δ; and plasmids: pWN43, Win1ΔN; and pWN46, Win1ΔN(K1149R).
MAPKKs have conserved serine/threonine and threonine residues between kinase subdomains VII and VIII, and phosphorylation of these residues plays a central role in the regulation of kinase activity (Alessi et al., 1994; Zheng and Guan 1994). Wis1 has such residues at positions 469 and 473, and they are essential for response to osmotic stress (Samejima et al., 1997). wis1-4 is an allele that has both Ser469 and Thr473 mutated to nonphosphorylatable glutamate residues. Cells carrying this allele are insensitive to overexpression of win1ΔN. The phosphotyrosine level on Spc1 remained at the same level in the wis1-4 mutant, irrespective of the expression level of win1ΔN (Figure 4), suggesting that phosphorylation at Ser469 and Thr473 of Wis1 is essential for the action of Win1.
Hyperactivation of Wis1 by phosphorylation is toxic to wild-type cells and inhibits colony formation (Samejima et al., 1997), as is overexpression of the wis1+ gene (Shiozaki and Russell, 1995). Consistent with the phosphotyrosine data shown above, overexpression of win1ΔN was toxic in wild-type cells, and colony formation was inhibited (Figure 4). In contrast, overexpression of mutant win1ΔN(K1149R) protein had no such effect, showing that increased kinase activity was responsible for the toxicity. The toxic phenotype was suppressed in wis1Δ and wis1-4 strains, suggesting that Win1 function requires phosphorylation of Wis1 at Ser469 and Thr473 (Figure 4). Taken together, these results suggest that Win1 phosphorylates these residues to activate Wis1.
win1-1 Mutation Site Lies in the New MAPKKK Coding Region
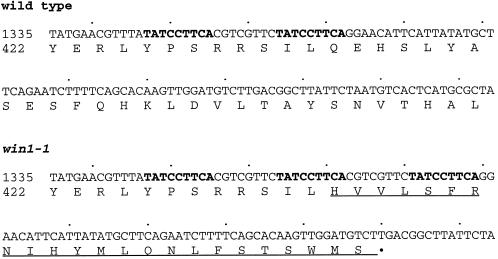
We asked whether the DNA from a win1-1 strain has a mutation in the ORF encoding the new MAPKKK. The ORF region was amplified by PCR from genomic DNA isolated from a win1-1 strain, and the PCR product was used as a template for DNA sequencing. An insertion of 16 nucleotides was found compared with the wild-type sequence. The wild-type sequence has a pair of repetitive sequences (TATCCTTCA) at positions 1347 … 1355 and 1364 … 1372 (Figure 5). In win1-1, the heptamer sequence, GTCGTTC, which is flanked by the TATCCTTCA repeat, is duplicated with an extra copy of TATCCTTCA in between (Figure 5). This duplication of 16 nucleotides results in a frameshift that replaces the carboxyl 1002 residues including the kinase domain with 23 irrelevant amino acids (Figure 5). The mutant protein thus has 456 residues in total, with the amino-terminal portion of the original 433 residues. The presence of such a mutation in win1-1 strain strongly suggests that the ORF is the win1+ gene. Moreover, the disruption of this ORF produced phenotypes very similar, if not identical, to win1-1 (see below).
Figure 5.
Mutation site of win1-1. The nucleotide sequences of part of the ORF of the MAPKKK in wild-type and win1-1 strains are shown with deduced amino acid sequences. The repetitive sequences are shown in bold. The mutant protein sequence generated by the frameshift is underlined.
Disruption of the MAPKKK Gene Is Sufficient to Give win1-1 Phenotype
The chromosomal region that encodes the last 278 residues (1159–1436) of the win1+ gene was replaced by the S. pombe ura4+ gene, removing most of the protein kinase catalytic domain from the genome. The resulting strain is viable, and in a cross between the deletion strain and win1-1, all of the 33 tetrads dissected were parental ditype, confirming very close linkage of the deletion mutation to win1-1. The chromosomal deletion is hereafter referred to as win1Δ. Several phenotypes conferred by the win1Δ mutation were examined and compared with the win1-1 mutant. Dividing win1Δ cells are longer than the wild type during growth on EMM (our unpublished observations), suggesting a mitotic delay as in win1-1 (Ogden and Fantes, 1986). The double mutant, cdc25-22 wee1-50 and the win1-1 single mutant can grow and make colonies at 36°C. However, the combination of the three mutations makes a cell unable to grow at this temperature, and the cell shows a cdc phenotype (Ogden and Fantes, 1986). The win1Δ mutation had a similar effect, and extremely elongated cells were observed with the triple mutant win1Δ cdc25-22 wee1-50 (our unpublished observations).
Consistent with the demonstration that the Win1 MAPKKK is an activator of Wis1 (Figures 3 and 4), the phosphotyrosine content of Spc1 in win1Δ was lower than in the wild-type strain (our unpublished observations), as in win1-1 (Samejima et al., 1997). Moreover, the toxicity of overexpression of wis1+ was alleviated in win1Δ strain just as in win1-1 and wis4Δ strains (our unpublished observations). The Wis1 kinase is activated when the wild-type cell is exposed to high osmolarity, and its substrate Spc1 is rapidly tyrosine phosphorylated (Millar et al., 1995; Shiozaki and Russell, 1995). On the contrary, the phosphotyrosine level remained low in both win1Δ and win1-1 strains (our unpublished observations; Samejima et al., 1997). This suggests that Win1 is required to activate Wis1 in response to high osmolarity.
Win1 and Wis4 Have Distinct Biological Roles in Stress Response
Previous reports have demonstrated that Wis4 phosphorylates and activates Wis1. However, the question of whether Wis4 transmits an osmostress signal to Wis1 is controversial, and the physiological function of Wis4 is not established (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997). We tested whether introduction of multiple copies of the wis4+ gene could suppress the phenotypes of win1-1 observed when the strain is challenged by high osmolarity.
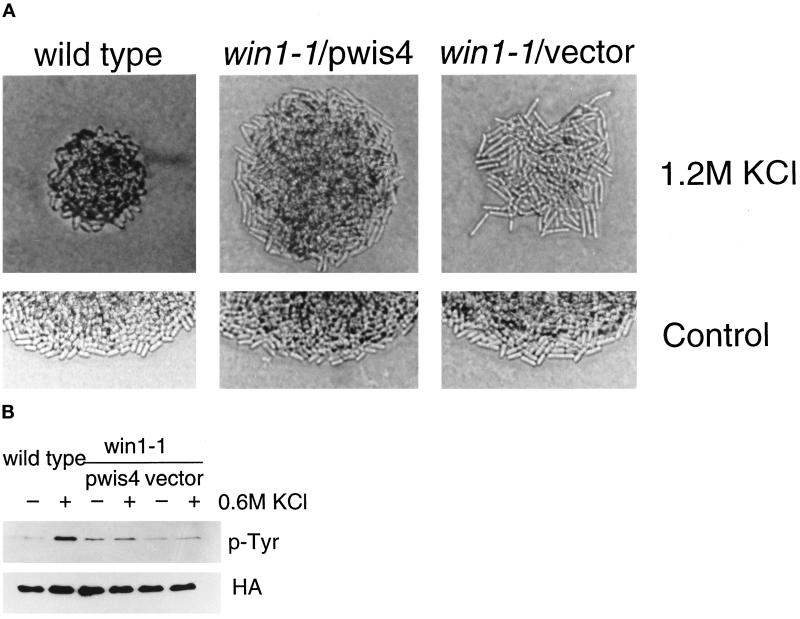
Wild-type cells undergo morphological changes on a high-osmolarity medium, just as when the wis1+ gene is overexpressed from a strong promoter or when wis1 is activated by ectopic overexpression of a truncated MAPKKK gene (Shiozaki and Russell, 1995; Samejima et al., 1997). Wild-type cells are short and swollen on medium containing 1.2 M KCl, whereas win1-1 cells are more elongated than on normal medium (Samejima et al., 1997; Figure 6A). The win1-1 strain carrying a wis4+ plasmid appears more similar to win1-1 than to the wild type on high-osmolarity medium, in that it retains the same width as on EMM and cell length is greater than on the control medium (Figure 6A). win1-1 cells carrying multiple copies of wis4+ are shorter than the control win1-1 cells in both conditions (with or without KCl). Thus a single copy of the win1+ gene (in wild-type cells) allows morphological change in response to high osmotic conditions. However, in the absence of win1+ function, even the presence of multiple copies of wis4+ does not restore this ability.
Figure 6.
wis4+ does not suppress the win1-1 defect under high-osmolarity conditions. The win1-1 strain carrying multiple copies of a genomic fragment containing the wis4+ gene was compared with the wild-type strain. (A) Cells in a colony were photographed after 3 d on EMM+ 1.2 M KCl or 2 d on EMM (control). (B) The phosphotyrosine content of Spc1 in wild-type or win1-1 strains determined by anti-phosphotyrosine antibody. The HA blot shows the relative amounts of HA-tagged Spc1. Cells were collected by filtration before or 10 min after exposure to 0.6 M KCl.
Another effect of the win1-1 mutation relevant to osmostress response is a change in the phosphotyrosine content of Spc1. In wild-type cells, Spc1 is rapidly tyrosine phosphorylated after exposure to 0.6 M KCl, whereas this response is lost in win1-1 cells. Although the presence of multiple copies of wis4+ in win1-1 cells raised the basal level of tyrosine phosphorylation, we observed no significant increase in response to KCl treatment (Figure 6B). This observation argues against a major role of wis4 in response to high osmolarity.
DISCUSSION
We have identified a second MAPKKK homologue of the Wis4 class in S. pombe. Several lines of evidence argue that it is encoded by the win1+ gene, previously only identified by the single win1-1 mutation. The tight genetic linkage between win1-1 and a marker integrated at the new MAPKKK gene (win1Δ) suggests that their chromosomal locations are very close. The genetic distance of 5 cM between win1-1 and a nearby marker, tps19, agrees well with the physical distance of 30–40 kb from tps19+ to the MAPKKK gene. It is the only gene on 3F9 that is not represented on any of the overlapping sets of cosmids, and the fact that 3F9, but no cosmids, suppresses the ts phenotype of the win1-1 cdc25-22 wee1-50 triple mutant suggests strongly that the MAPKKK gene is win1. Further supporting evidence is the discovery of a frameshift mutation within the MAPKKK ORF in the win1-1 strain, which would cause a truncation eliminating the carboxyl-terminal kinase domain. Consistent with this, the phenotypes of win1-1 and win1Δ are almost identical. Furthermore, all the phenotypes of win1-1 can be very well explained by Win1 being an MAPKKK that activates Wis1. Further work will be needed for a full characterization of Win1: in particular the isolation of a full-length clone, which has so far eluded us, for reasons discussed below.
In a previous report, we showed that Wis1 can be activated by phosphorylation at its conserved activation residues, presumably because of the action of one or more MAPKKKs, one of which is Wis4. We also reported that Wis4 could not account for the response to osmotic stress but showed that the response was almost abolished by a mutation in win1, whose molecular identity was not then known. We now show that Win1 is an MAPKKK homologous to Wis4. This explains very well that although phosphorylation of Wis1 is essential for activation of Wis1 in response to high osmolarity, wis4+ is not required, and that Win1 activates Wis1 in parallel to Wis4. S. pombe Mcs4, the presumed receptor of a bacterial-like two-component phospho-relay system, has been reported to act upstream of the Wis4–Wis1–Spc1 pathway (Cottarel, 1997; Shieh et al., 1997; Shiozaki et al., 1997). By analogy with the HOG pathway in S. cerevisiae where a similar two-component system is implicated in detection of high osmolarity and in the activation of two MAPKKKs (Maeda et al., 1994), it might be expected that Wis4 plays a role in transmitting osmostress signals to the Wis1–Spc1 pathway. However, there is so far no evidence for wis4+ playing a major role in osmostress signaling in wild-type cells. In addition, the data we present here show that wis4+ cannot suppress the win1-1 phenotypic defects that are manifest under conditions of high osmolarity. A model that summarizes these and other observations is shown in Figure 7.
Figure 7.
Stress response pathway in fission yeast. Win1 MAPKKK phosphorylates Wis1 MAPKK in response to high osomolarity. Heat and oxidative stresses activate the pathway independently of Win1 and Wis4 MAPKKKs (Samejima et al., 1997). Spc1 is also known as StyI or Phh1. Wis4 is synonymous with Wak1 and Wik1.
The strategies for detection of high osmolarity and transduction of these signals vary in different organisms. In Saccharomyces cerevisiae, a pair of MAPKKKs, Ssk2p and Ssk22p, are required to transduce the signal from the bacterial-like two-component system, which uses phosphate transfer from histidine to aspartate, to the Pbs2 MAPKK homologue (Parkinson and Kofoid, 1992; Maeda et al., 1994). Pbs2 can alternatively be activated by the Ste11 MAPKKK in an unknown way that does not involve the SLN1–YPD1–SSK1 two-component system (Posas and Saito, 1997), indicating that a two-component phospho-relay system is not the only way to detect and transduce high-osmolarity signals. In mammalian cells, multimerization of receptor tyrosine kinases such as the epidermal growth factor, interleukin 1, and tumor necrosis factor receptors in response to high osmotic stress leads to activation of a c-jun NH2-terminal kinase (JNK) via MEKK-1, but no evidence for the presence of a bacterial-like two-component system has been reported (Rosette and Karin, 1996). A better understanding of the Win1 MAPKKK is expected to pave the way to understanding the mechanism of detection and transduction of high-osmolarity signals in S. pombe.
The win1-1 mutation contains an insertion of 16 nucleotides within the amino-terminal domain. The win1-1 mutant was obtained fortuitously during an attempt to isolate plasmids that suppress wee1-50 and is probably a spontaneous mutation (Ogden and Fantes, 1986). It appears that the extra sequence has arisen by duplication of a tandemly repeated sequence and the short sequence between the repeats. We observe an occasional reversion of win1-1 phenotype, which might be explained by a similar oblique recombination event reversing the original mutation. The tandem duplication present in the win1+ gene may help explain why it has proved so difficult to obtain full-length clones either by screening of several libraries (Warbrick and Fantes, 1992) or by attempting to subclone the activity from phage 3F9 (Samejima, unpublished observations). Propagation of tandem repeats in E. coli can lead to recombination and loss of nucleotide sequence, which in the case of win1+ would cause a frameshift and loss of function. However there may be other factors involved, because both of the ordered cosmid libraries constructed have a gap at the same location, which corresponds to the win1+ gene.
Until the molecular identity of the win1+ gene and nature of the win1-1 mutation were known, the phenotype of the triple mutant could not be fully interpreted. We have shown that Win1 is an MAPKKK that activates the Wis1 MAPKK, and that win1-1 is a loss of function mutation. These findings account for the basis of our previous observation that Wis1 is less active in the win1-1 mutant (Samejima et al., 1997). Reduced Wis1 activity in the win1-1 mutant is one factor responsible for the cell cycle defect of wee1-50 cdc25-22 win1-1 at 35°C, because cdc25-22 wee1-50 is viable at this temperature. It is reduced Wis1 activity rather than the the specific loss of Win1 activity that seems to be important, because the combination of cdc25-22 wee1-50 with other mutations that reduce the level of Wis1 activity (e.g., spc1Δ and wis4Δ) all result in similar phenotypes (Shiozaki and Russell, 1995; our unpublished data). Because wee1-50 win1-1 is viable at 35°C, lethality of the triple mutant also requires the cdc25-22 defect. By analogy with the synthetic lethality of the cdc25-22 mutation with wis1Δ or spc1Δ (Shiozaki and Russell, 1995, 1996), the lethality of wee1-50 cdc25-22 win1-1 at 35°C is probably a combined result of two defects: one in the Wis1 pathway and the other in Cdc25 activity. This rather simplified view of the phenotype might be useful in understanding the suppression of wee1-50 cdc25-22 win1-1 by the various wis genes. Suppression could occur either by alleviation of the cdc25-22 mutation or by gain of Wis1 activity to compensate for the partial loss attributable to the win1-1 mutation. Overexpression of the various wis genes might suppress by either mechanism.
The win1-1 mutant gene encodes a truncated protein lacking a kinase domain, and it is therefore impossible for wis genes to restore Win1 kinase activity per se in win1-1 mutants. Enhanced Wis1 kinase activity, however, can compensate for loss of win1 function. For instance, overexpression of wis1+ or wis4+ increases the total kinase activity of Wis1 available and substitutes for the missing contribution from win1+ under unstressed conditions. Unlike the case for wis1+ and wis4+, the underlying mechanism for suppression by other wis genes is not evident. The wis2+ gene encodes a cyclophilin of the Cyp-40 class (Weisman et al., 1996). Its predicted peptidyl-prolyl isomerase activity may be required for activation or stabilization of one or more of the kinases in the osmosensing cascade, because some protein kinases (e.g., Wee1 and Src) need a chaperone complex for their function in vivo (Aligue et al., 1994; Gerber et al., 1995; Dey et al., 1996). It is, however, more plausible that wis2+ (and wis3+) suppress the reduced activity of the the cdc25-22 mutant protein, because they suppress other mutations in a cdc25-22 wee1-50 background, and neither wis2+ nor wis3+ suppresses the win1-1 single mutant (Warbrick and Fantes, 1992).
The win1-1 cdc25-22 wee1-50 mutant provides a rather sensitive way to isolate genes in two apparently distinct mitotic inducing systems, the wis1 pathway and Cdc25 activation. The effect of a slight enhancement of Cdc25 phosphatase activity in a cdc25-22 mutant strain might be more pronounced in a wee1 mutant background, which might increase the sensitivity of cells to a weak suppressor. However, cdc25-22 wee1-50 cells are able to grow at all temperatures, so that no selection for suppressors of this type is available. The partial reduction of Wis1 activity by win1-1 in cdc25-22 wee1-50 win1-1 cells appears to offer suitable conditions for selection of suppressors. Another advantage of screening for regulators of Cdc25 in a wee1 mutant background over a simple screen with the cdc25-22 single mutant is that the former approach should avoid isolation of suppressors such as nim1+, which suppress the cdc25-22 defect by an indirect mechanism. For these reasons, this genetic screen might contribute to our understanding of the complex regulation of Cdc25 phosphatase activity (Kumagai and Dunphy, 1992; Kovelman and Russell, 1996; Furnari et al., 1997; Peng et al., 1997). It is likely (although not proven) that Wis2 and Wis3 act on Cdc25 or one of its regulators, and further characterization of these proteins should be highly informative. With regard to the wis1 pathway, a more exhaustive screen for multicopy suppressors of the triple mutant might identify the putative cell cycle effector(s) of Spc1, in addition to the activators of Spc1 such as Wis1 and Wis4 that have been isolated to date.
ACKNOWLEDGMENTS
We thank Nicola Preston and Vicky Clark for DNA sequencing, Maria Victoria Zarate for help at the very early stage of cloning of tps19+ gene, Elmer Maier and Mitsuhiro Yanagida for information on S. pombe ordered cosmid/P1 phage libraries, and Steve Sedgwick for a gift of a transposon kit. Many thanks go to Joan Davidson and Aileen Greig for technical assistance and Yasuhisa Adachi, Bill Earnshaw, and Colin Gordon for generous encouragement. This work was supported by grants from the Wellcome Trust, Cancer Research Campain (to P.A.F.), and the Daiwa Anglo-Japanese Foundation (to I.S.). I.S. was a recipient of a Travelling Research Fellowship from the Wellcome Trust.
REFERENCES
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. [Google Scholar]
- Aligue R, Akhavan-Niak H, Russell P. A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J. 1994;13:6099–6106. doi: 10.1002/j.1460-2075.1994.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Cairns BR, Ramer SW, Kornberg RD. Order of action of components in the yeast pheromone response pathway revealed with a dominant allele of the STE11 kinse and the multiple phosphorylation of the STE7 kinase. Genes Dev. 1992;6:1305–1318. doi: 10.1101/gad.6.7.1305. [DOI] [PubMed] [Google Scholar]
- Cottarel G. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics. 1997;147:1043–1051. doi: 10.1093/genetics/147.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B, Lightbody JJ, Boschelli F. CDC37 is required for p60v-src activity in yeast. Mol Biol Cell. 1996;7:1405–1417. doi: 10.1091/mbc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Derijard B, Wu I-H, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Gerber MR, Farrell A, Deshaies RJ, Herskowitz I, Morgan DO. Cdc37 is required for association of the protein kinase Cdc28 with G1 and mitotic cyclins. Proc Natl Acad Sci USA. 1995;92:4651–4655. doi: 10.1073/pnas.92.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Kohli J, Murray J, Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using ura4+ gene as a selectable marker. Mol Gen Genet. 1998;215:81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Hoheisel JD, Maier E, Mott R, McCarthy L, Grigoriev AV, Schalkwyk LC, Nizetic D, Francis F, Lehrach H. High resolution cosmid and P1 maps spanning the 14 Mb genome of the fission yeast S. pombe. Cell. 1993;73:109–120. doi: 10.1016/0092-8674(93)90164-l. [DOI] [PubMed] [Google Scholar]
- Kato T, Jr, Okazaki K, Murakami H, Stettler S, Fantes PA, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kovelman R, Russell P. Stockpiling of Cdc25 during a DNA replication checkpoint arrest in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:86–93. doi: 10.1128/mcb.16.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai A, Dunphy WG. Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell. 1992;70:139–151. doi: 10.1016/0092-8674(92)90540-s. [DOI] [PubMed] [Google Scholar]
- Lee KS, Levin DE. Dominant mutations in a gene encoding a putative protein kinase (BCK1) bypass the requirement for a Saccharomyces cerevisiae protein kinase C homolog. Mol Cell Biol. 1992;12:172–182. doi: 10.1128/mcb.12.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeill SA, Fantes PA. Controlling entry into mitosis in fission yeast. In: Hutchison C, Glover DM, editors. Cell Cycle Control. Oxford, United Kingdom: IRL Press; 1995. pp. 63–105. [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast-highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Mizukami T, et al. A 13 kb resolution cosmid map of the 14 Mb fission yeast genome by nonrandom sequence-tagged site mapping. Cell. 1993;73:121–132. doi: 10.1016/0092-8674(93)90165-m. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Conlon FL, Manzanares M, Millar JBA, Kanuga N, Sharpe J, Krumlauf R, Smith JC, Sedgwick SG. Transposon tools for recombinant DNA manipulation: characterization of transcriptional regulators from yeast, Xenopus, and mouse. Proc Natl Acad Sci USA. 1996;93:2801–2806. doi: 10.1073/pnas.93.7.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden JE, Fantes PA. Isolation of a novel type of mutation in the mitotic control of Schizosaccharomyces pombe whose phenotypic expression is dependent on the genetic background and nutritional environment. Curr Genet. 1986;10:509–514. doi: 10.1007/BF00447384. [DOI] [PubMed] [Google Scholar]
- Parkinson JS, Kofoid EC. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Peng C-Y, Graves PR, Thoma RS, Wu Z, Shaw AS, Piwnica-Worms H. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–1505. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of fission yeast stress-activated MAP kinase pathway. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Kapitza P, Gutz H. Switching genes in Schizosaccharomyces pombe: their influence on cell viability and recombination. Curr Genet. 1987;11:303–308. [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan B, Makino K, Millar JBA. The Mcs4 response regulator co-ordinately controls the stress activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–417. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BJ, Rhodes N, Errede B, Sprague GF., Jr Constitutive mutants of the protein kinase STE11 activate the yeast pheromone response pathway in the absence of the G-protein. Genes Dev. 1992;6:1293–1304. doi: 10.1101/gad.6.7.1293. [DOI] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. The wis1 protein kinase is a dose-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. Five novel elements involved in the regulation of mitosis in fission yeast. Mol Gen Genet. 1992;232:440–446. doi: 10.1007/BF00266249. [DOI] [PubMed] [Google Scholar]
- Weisman R, Creanor J, Fantes P. A multicopy suppressor of a cell cycle defect in S. pombe encodes a heat shock-inducible 40 kDa cyclophilin-like protein. EMBO J. 1996;15:447–456. [PMC free article] [PubMed] [Google Scholar]
- Zheng C-F, Guan K-L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994;13:1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]