Abstract
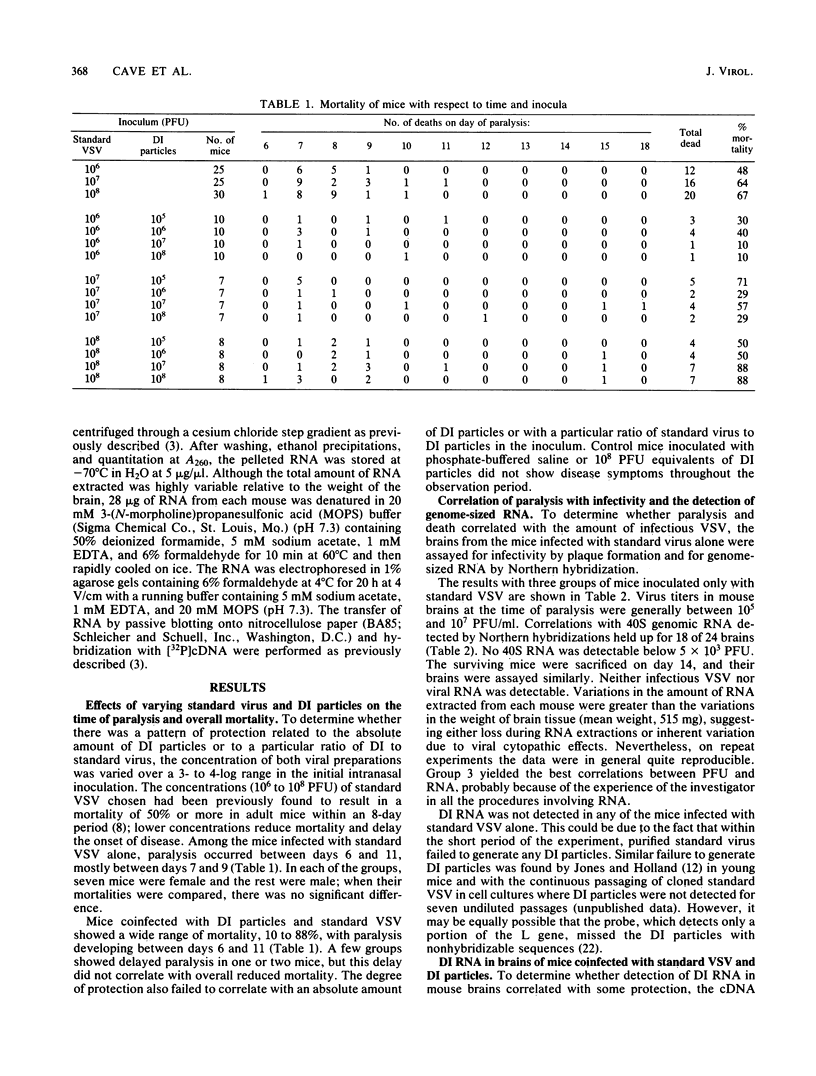
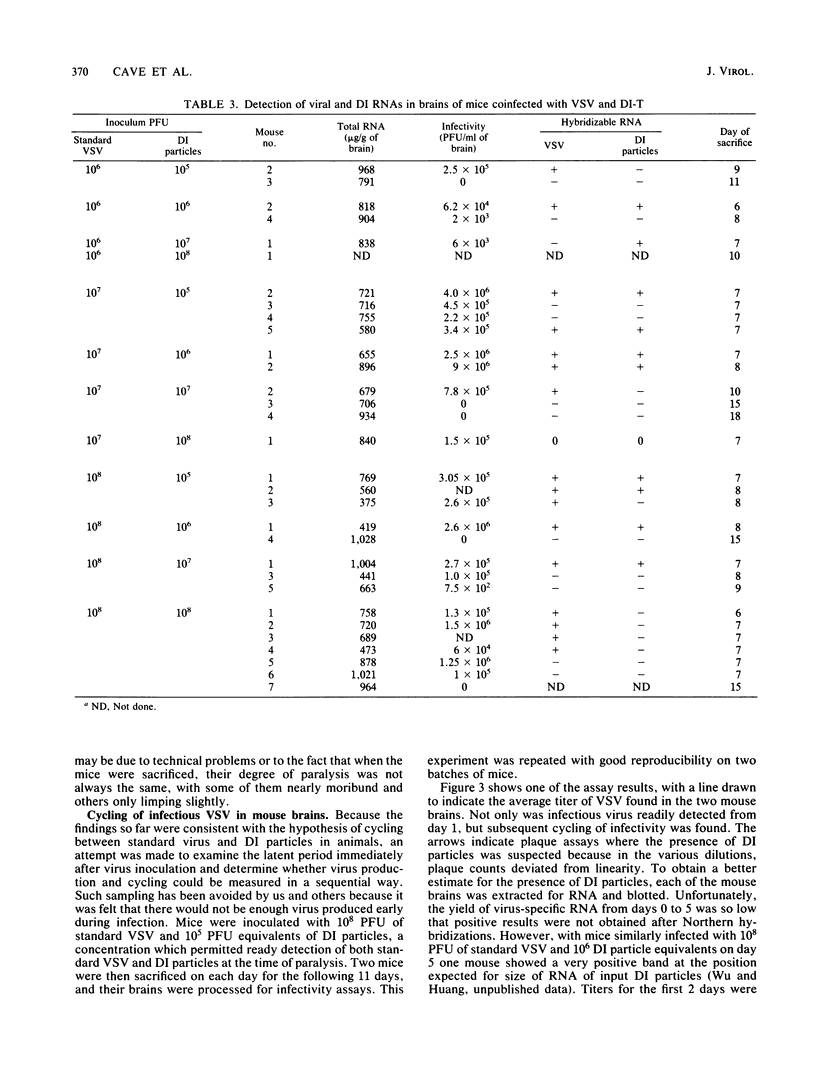
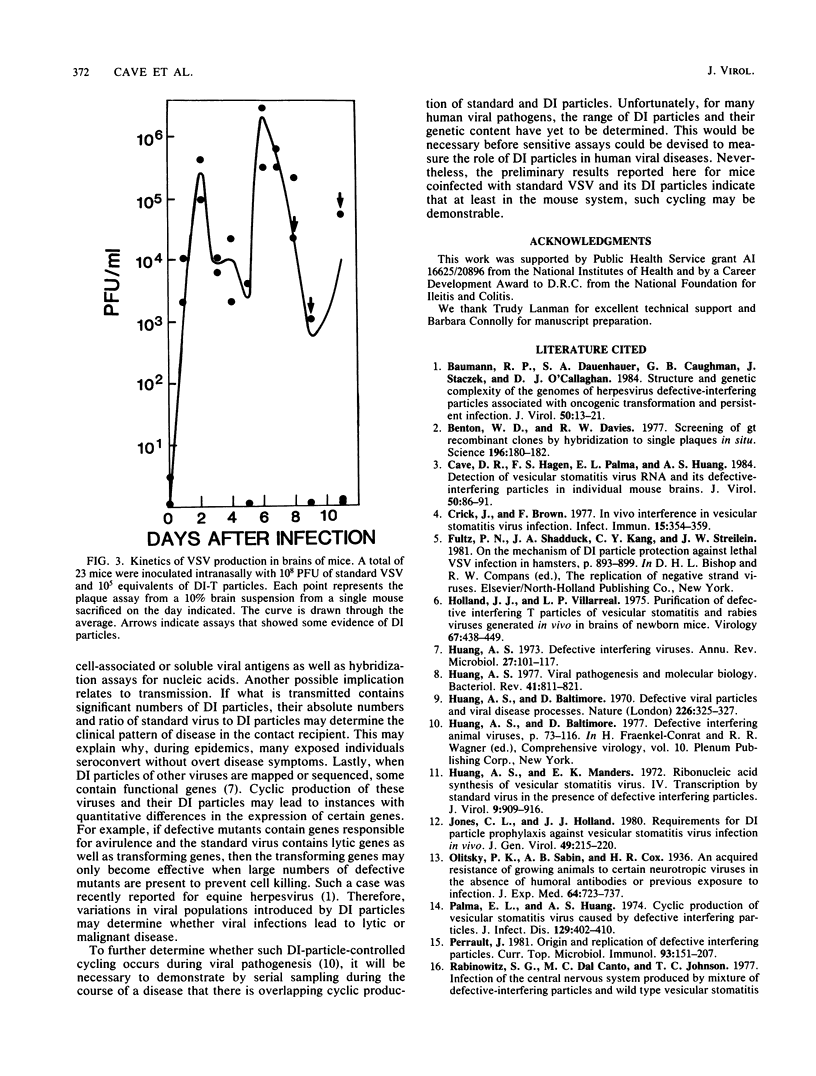
To determine whether defective interfering (DI) particles modulate virulence by initiating a cyclic pattern of virus growth in vivo, adult mice were infected with vesicular stomatitis virus (VSV), both with and without DI particles. A total of 184 mice divided into groups were inoculated intranasally. A majority of mice inoculated only with standard VSV developed paralysis, most of them between days 7 and 9. The addition of DI particles altered the development of paralysis in several ways. When there was significant protection, a few still became paralyzed on days 7 and 9. When overall mortality was unaffected or even slightly increased, the majority of mice became paralyzed between days 7 and 9 as well. Protection could not be predicted based on a single ratio of standard VSV to DI particles or on the absolute amount of DI particles inoculated. Infectious virus recovered from mouse brains at the time of paralysis and incipient death showed considerable variation, although the titer in a majority of the animals was between 10(5) and 10(7) PFU/ml. When the brains of these paralyzed mice were examined for hybridizable VSV RNA, the detection of standard VSV RNA correlated well with infectivity. The amount of DI RNA in the coinfected mice was more variable and independent of the amount of 40S RNA, although DI RNA was usually found when standard RNA was present. Survivors examined between days 14 and 21 did not contain infectious virus or any detectable viral RNA in their brains. Because these results were consistent with the hypothesis of viral cycling in vivo, rather than a gradual accumulation of total infectious virus, mice were coinfected with 10(8) PFU of standard VSV and 10(5) PFU equivalents of DI particles and sacrificed daily thereafter, irrespective of whether they developed paralysis. Infectivity measurements indicated a reproducible cycling pattern of VSV in the mouse brains with a periodicity of about 5 days. This cycling and the detection of DI RNA in brains several days after intranasal inoculation suggest that there is a dynamic continuous interaction between standard VSV and its DI particle beyond the initial site of replication as the virus population spreads into the host animal. Such cycling of virus production before the full development of specific immune responses from the host may have important implications for viral diagnostics and disease transmission.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann R. P., Dauenhauer S. A., Caughman G. B., Staczek J., O'Callaghan D. J. Structure and genetic complexity of the genomes of herpesvirus defective-interfering particles associated with oncogenic transformation and persistent infection. J Virol. 1984 Apr;50(1):13–21. doi: 10.1128/jvi.50.1.13-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Cave D. R., Hagen F. S., Palma E. L., Huang A. S. Detection of vesicular stomatitis virus RNA and its defective-interfering particles in individual mouse brains. J Virol. 1984 Apr;50(1):86–91. doi: 10.1128/jvi.50.1.86-91.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick J., Brown F. In vivo interference in vesicular stomatitis virus infection. Infect Immun. 1977 Feb;15(2):354–359. doi: 10.1128/iai.15.2.354-359.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P. Purification of defective interfering T particles of vesicular stomatitis and rabies viruses generated in vivo in brains of newborn mice. Virology. 1975 Oct;67(2):438–449. doi: 10.1016/0042-6822(75)90445-6. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S. Viral pathogenesis and molecular biology. Bacteriol Rev. 1977 Dec;41(4):811–821. doi: 10.1128/br.41.4.811-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. L., Holland J. J. Requirements for DI particle prophylaxis against vesicular stomatitis virus infection in vivo. J Gen Virol. 1980 Jul;49(1):215–220. doi: 10.1099/0022-1317-49-1-215. [DOI] [PubMed] [Google Scholar]
- Palma E. L., Huang A. Cyclic production of vesicular stomatitis virus caused by defective interfering particles. J Infect Dis. 1974 Apr;129(4):402–410. doi: 10.1093/infdis/129.4.402. [DOI] [PubMed] [Google Scholar]
- Perrault J. Origin and replication of defective interfering particles. Curr Top Microbiol Immunol. 1981;93:151–207. doi: 10.1007/978-3-642-68123-3_7. [DOI] [PubMed] [Google Scholar]
- Rao D. D., Huang A. S. Interference among defective interfering particles of vesicular stomatitis virus. J Virol. 1982 Jan;41(1):210–221. doi: 10.1128/jvi.41.1.210-221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Sinarachatanant P., Huang A. S. Effects of temperature and antibody on the cyclid growth of vesicular stomatitis virus. J Virol. 1977 Jan;21(1):161–167. doi: 10.1128/jvi.21.1.161-167.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



