Abstract
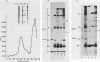
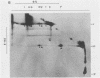
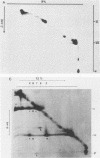
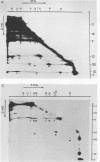
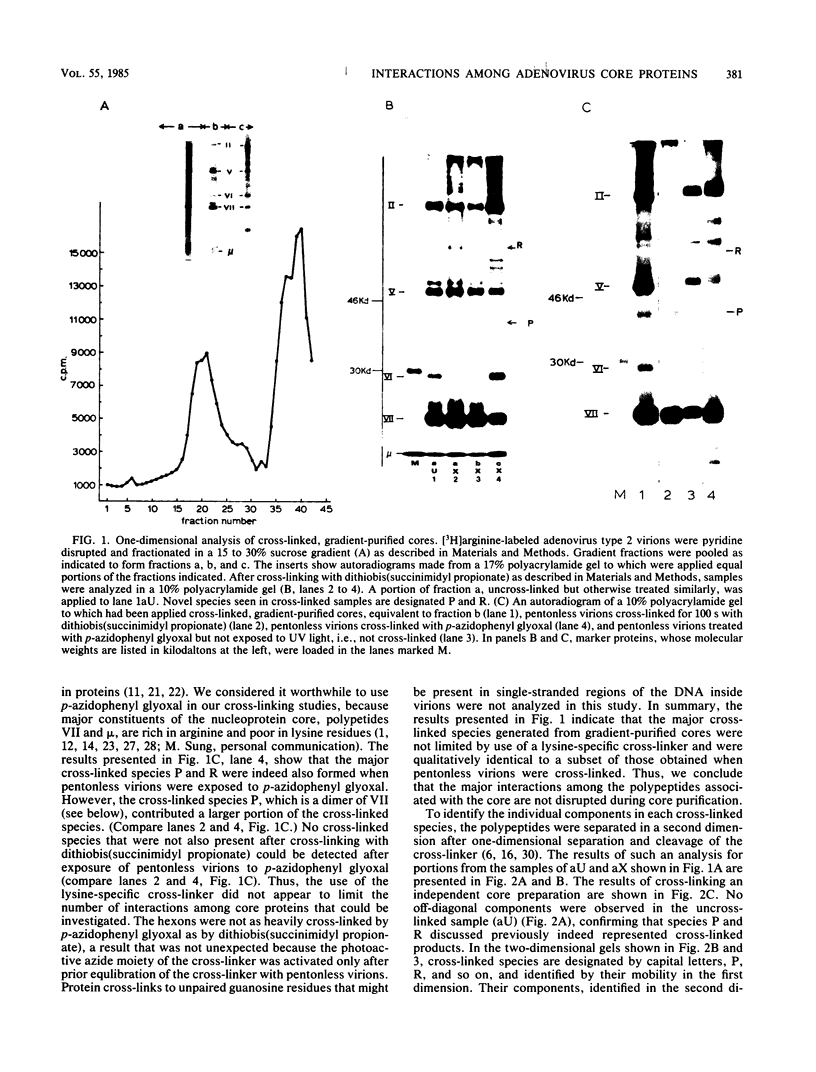
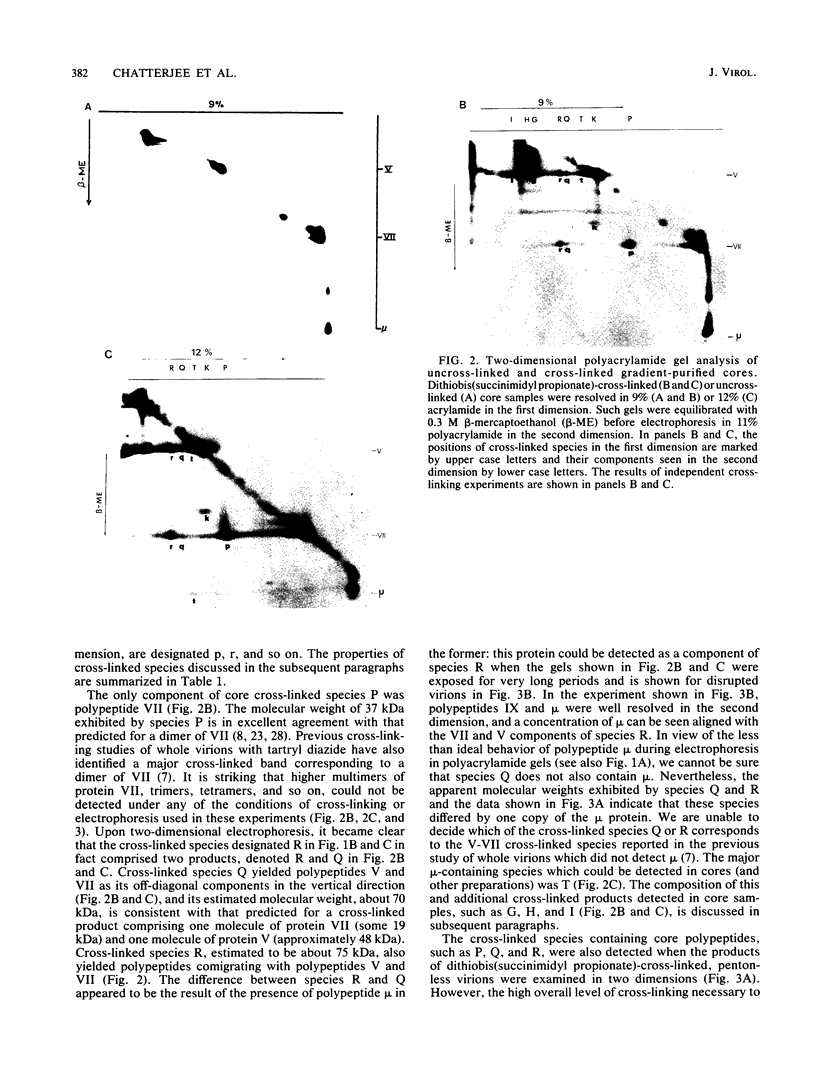
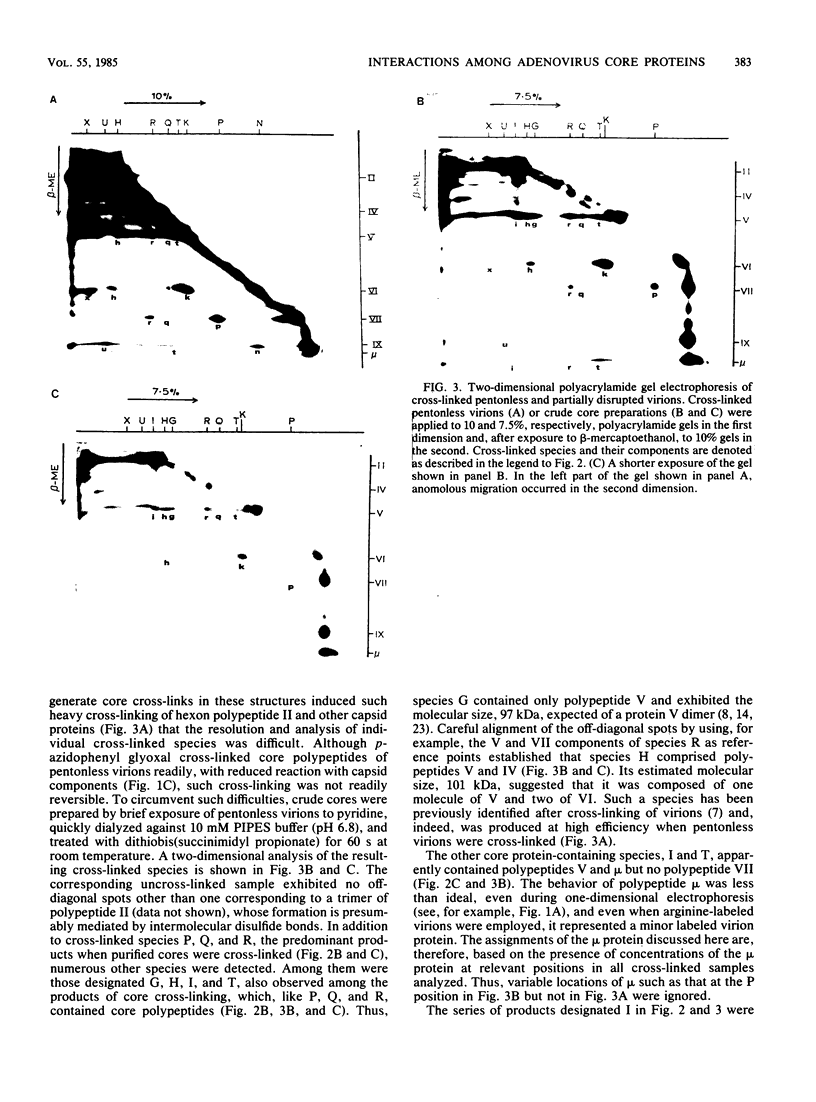
Interactions among the three adenovirus core polypeptides V, VII, and mu were examined, using the reversible chemical cross-linker dithiobis(succinimidyl propionate) and two-dimensional polyacrylamide gel electrophoresis. Cross-linked species obtained from gradient-purified adenovirus type 2 cores were well represented among the cross-linked products of pentonless virions and crude core preparations. The more efficiently formed cross-linked core species were also identified with the arginine-specific cross-linker, p-azidophenyl glyoxal. In addition to dimers of polypeptides V and VII, efficient cross-linking of V to VII, V to mu, and VII to V to mu was detected in adenovirus cores. Notably absent were cross-linked species corresponding to higher multimers of polypeptide VII. A major core-capsid interaction appeared to be via the association of polypeptide V with a dimer of polypeptide VI.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Lager M., Yeh-kai L., Pettersson U. Genes encoding the core proteins of adenovirus type 2. J Biol Chem. 1984 Nov 25;259(22):13980–13985. [PubMed] [Google Scholar]
- Binger M. H., Flint S. J., Rekosh D. M. Expression of the gene encoding the adenovirus DNA terminal protein precursor in productively infected and transformed cells. J Virol. 1982 May;42(2):488–501. doi: 10.1128/jvi.42.2.488-501.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglia C. L., Flint S. J. Effects of adenovirus infection on rRNA synthesis and maturation in HeLa cells. Mol Cell Biol. 1983 Apr;3(4):662–671. doi: 10.1128/mcb.3.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Glass J. D., Pelzig M. Reversible modification of arginine residues with glyoxal. Biochem Biophys Res Commun. 1978 Mar 30;81(2):527–531. doi: 10.1016/0006-291x(78)91566-8. [DOI] [PubMed] [Google Scholar]
- Hosokawa K., Sung M. T. Isolation and characterization of an extremely basic protein from adenovirus type 5. J Virol. 1976 Mar;17(3):924–934. doi: 10.1128/jvi.17.3.924-934.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Lischwe M. A., Sung M. T. A histone-like protein from adenovirus chromatin. Nature. 1977 Jun 9;267(5611):552–554. doi: 10.1038/267552a0. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Structure of adenovirus chromatin. Biochim Biophys Acta. 1982 Jan 26;696(1):76–86. doi: 10.1016/0167-4781(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. Structural elements in adenovirus cores. Evidence for a "core shell" and linear structures in "relaxed" cores. Arch Virol. 1979;62(2):101–116. doi: 10.1007/BF01318063. [DOI] [PubMed] [Google Scholar]
- Nermut M. V. The architecture of adenoviruses: recent views and problems: Brief review. Arch Virol. 1980;64(3):175–196. doi: 10.1007/BF01322699. [DOI] [PubMed] [Google Scholar]
- Patthy L., Smith E. L. Reversible modification of arginine residues. Application to sequence studies by restriction of tryptic hydrolysis to lysine residues. J Biol Chem. 1975 Jan 25;250(2):557–564. [PubMed] [Google Scholar]
- Politz S. M., Noller H. F., McWhirter P. D. Ribonucleic acid-protein cross-linking in Escherichia coli ribosomes: (4-azidophenyl)glyoxal, a novel heterobifunctional reagent. Biochemistry. 1981 Jan 20;20(2):372–378. doi: 10.1021/bi00505a023. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U. Structural proteins of adenoviruses. VII. Purification and properties of an arginine-rich core protein from adenovirus type 2 and type 3. Virology. 1971 Aug;45(2):364–373. doi: 10.1016/0042-6822(71)90337-0. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Sung M. T., Cao T. M., Coleman R. T., Budelier K. A. Gene and protein sequences of adenovirus protein VII, a hybrid basic chromosomal protein. Proc Natl Acad Sci U S A. 1983 May;80(10):2902–2906. doi: 10.1073/pnas.80.10.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- Traut R. R., Bollen A., Sun T. T., Hershey J. W., Sundberg J., Pierce L. R. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973 Aug 14;12(17):3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Rogers A. E., Flint S. J. The structure of nucleoprotein cores released from adenovirions. Nucleic Acids Res. 1983 Jan 25;11(2):441–460. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]