Abstract
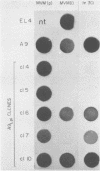
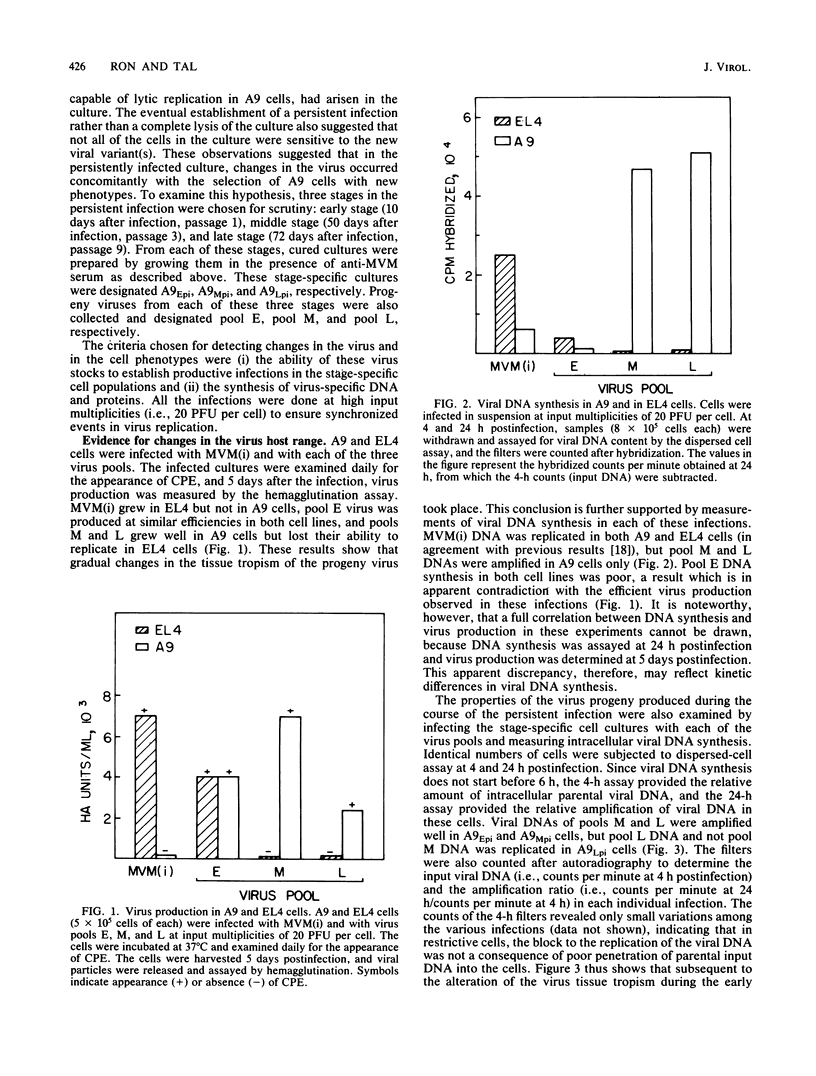
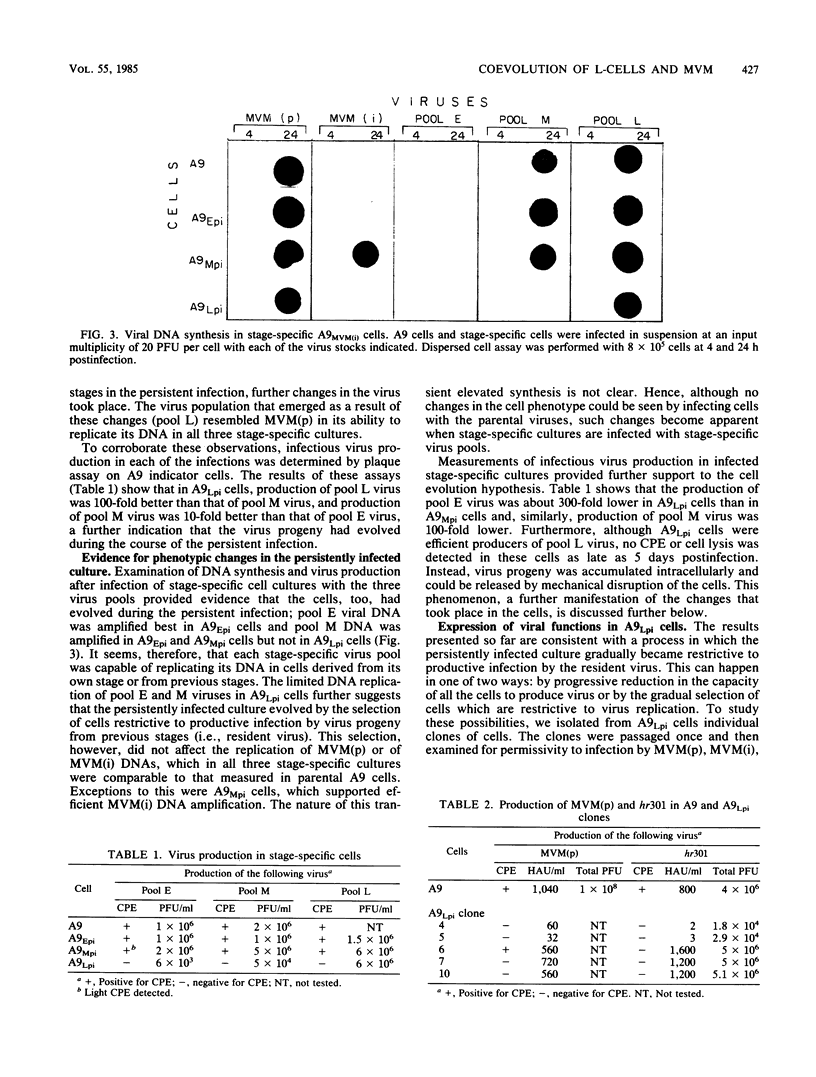
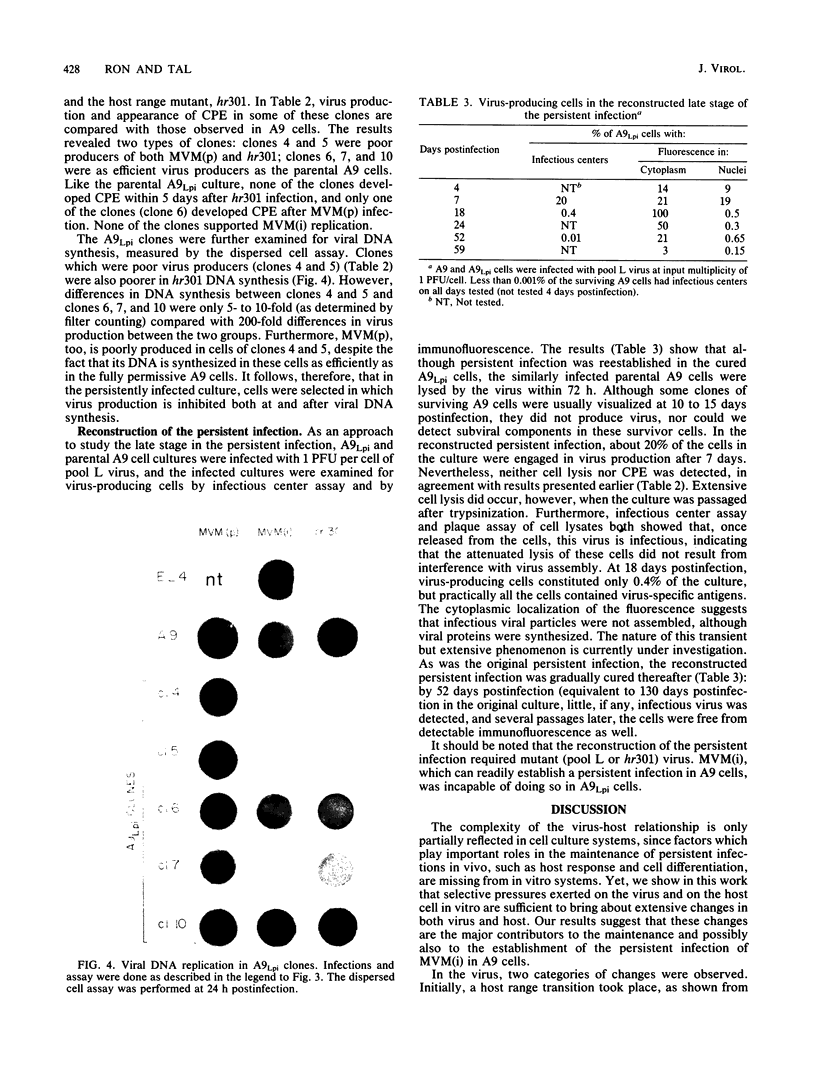
Infection of L-cells with minute virus of mice (i), a lymphotropic strain of minute virus of mice, resulted in the emergence of host range mutant viruses capable of a lytic infection that destroys the initially restrictive parental cells. Despite that, the culture was not lysed completely; instead, a persistent infection resulted which lasted at least 150 days. Throughout the persistent infection, extensive changes occurred in both the tissue tropism of the progeny virus and in the phenotypic properties of the cells. Mutant cells were selected which were increasingly restrictive to the replication of the resident virus, but concomitant changes in the virus enabled it to replicate in a subpopulation of the restrictive cells. The persistent infection could be reconstructed by infection of mutant cells with mutant virus; in contrast, neither infection of parental cells with mutant virus nor infection of mutant cells with parental virus led to persistence. On the basis of these results, we suggest that virus-cell coevolution provides the primary mechanism for the initiation and the maintenance of the persistent infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Canning W. M., Kauffman R. S., Sharpe A. H., Hallum J. V., Fields B. N. Role of the host cell in persistent viral infection: coevolution of L cells and reovoirus during persistent infection. Cell. 1981 Aug;25(2):325–332. doi: 10.1016/0092-8674(81)90050-7. [DOI] [PubMed] [Google Scholar]
- Bonnard G. D., Manders E. K., Campbell D. A., Jr, Herberman R. B., Collins M. J., Jr Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. Evidence for minute virus of mice causing the inhibition. J Exp Med. 1976 Jan 1;143(1):187–205. doi: 10.1084/jem.143.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Lavi S., Etkin S. Carcinogen-mediated induction of SV40 DNA synthesis in SV40 transformed Chinese hamster embryo cells. Carcinogenesis. 1981;2(5):417–423. doi: 10.1093/carcin/2.5.417. [DOI] [PubMed] [Google Scholar]
- Mathews J. H., Vorndam A. V. Interferon-mediated persistent infection of Saint Louis encephalitis virus in a reptilian cell line. J Gen Virol. 1982 Aug;61(Pt 2):177–186. doi: 10.1099/0022-1317-61-2-177. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y. Studies of L cells persistently infected with VSV: factors involved in the regulation of persistent infection. J Gen Virol. 1977 May;35(2):265–279. doi: 10.1099/0022-1317-35-2-265. [DOI] [PubMed] [Google Scholar]
- Ron D., Tattersall P., Tal J. Formation of a host range mutant of the lymphotropic strain of minute virus of mice during persistent infection in mouse L cells. J Virol. 1984 Oct;52(1):63–69. doi: 10.1128/jvi.52.1.63-69.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M., Stobo J. D., Green I. Immunoglobulin and theta-bearing murine leukemias and lymphomas. J Immunol. 1972 May;108(5):1146–1151. [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., HABEL K. Virus-cell relationship in a carrier culture of HeLa cells and Coxsackie A9 virus. Virology. 1959 Jan;7(1):28–44. doi: 10.1016/0042-6822(59)90175-8. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Integrated genomes of animal viruses. Annu Rev Biochem. 1980;49:197–226. doi: 10.1146/annurev.bi.49.070180.001213. [DOI] [PubMed] [Google Scholar]