Abstract
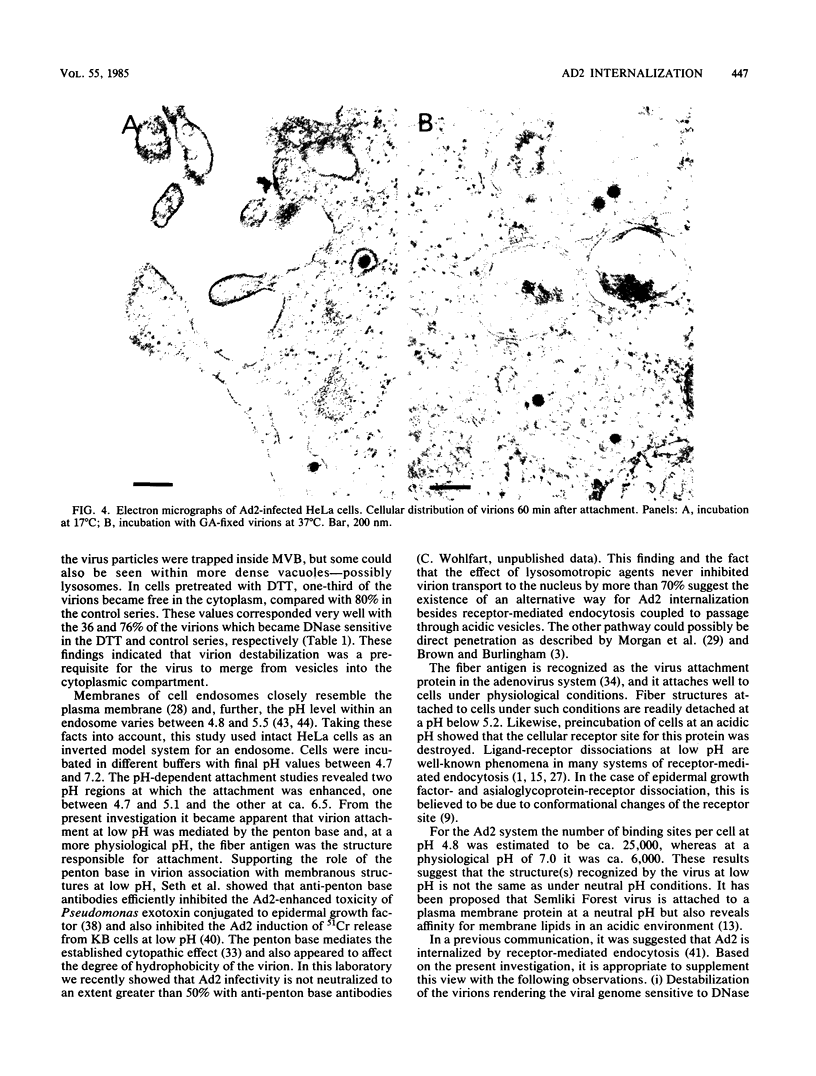
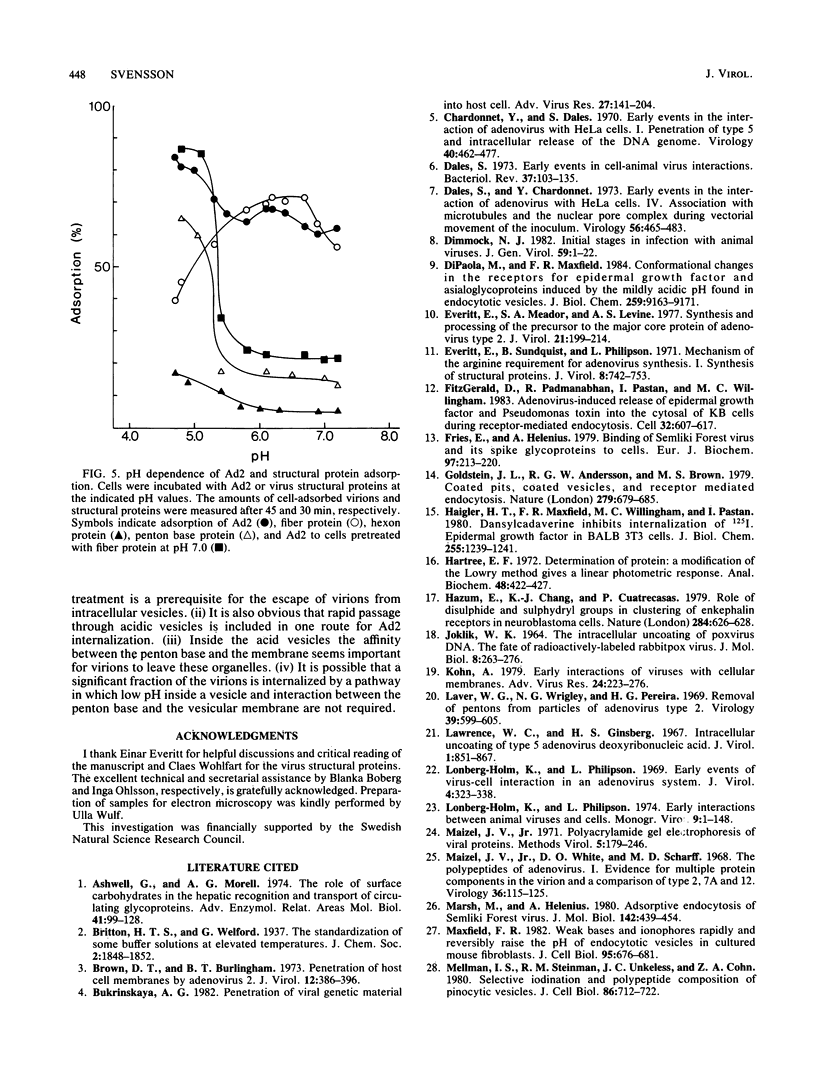
In this investigation, the early period of adenovirus type 2 (Ad2)-HeLa cell interaction was analyzed by electron microscopy and biochemical techniques. Events observed in this period ranged from the disappearance of virions from the cell surface to their subsequent association with the cell nucleus. Destabilization of the virions attached to the intact cell was necessary for virions to escape from intracellular vesicles. Strong temperature dependence and rapid escape from a vesicular compartment were shown in temporal kinetic experiments. These vesicles appeared to be acidic, since lysosomotropic agents partly inhibited the release of virions from vesicles. Studies of Ad2 binding to cells in buffers of different pH values suggested that adenovirus binds to cells by two different mechanisms. At low pH the binding was most probably mediated by the penton base and at neutral pH by the fiber protein. The number of receptor sites per cell was 25,000 and 6,000 at low and neutral pH, respectively. This study suggests that the low-pH affinity between the penton base and a vesicular membrane is important inside acid vesicles when Ad2 quickly enters the cytoplasm. However, a significant fraction of the virions was possibly internalized by a pathway not requiring a passage through such vesicles.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chardonnet Y., Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970 Mar;40(3):462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- Dales S., Chardonnet Y. Early events in the interaction of adenoviruses with HeLa cells. IV. Association with microtubules and the nuclear pore complex during vectorial movement of the inoculum. Virology. 1973 Dec;56(2):465–483. doi: 10.1016/0042-6822(73)90050-0. [DOI] [PubMed] [Google Scholar]
- Dales S. Early events in cell-animal virus interactions. Bacteriol Rev. 1973 Jun;37(2):103–135. doi: 10.1128/br.37.2.103-135.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPaola M., Maxfield F. R. Conformational changes in the receptors for epidermal growth factor and asialoglycoproteins induced by the mildly acidic pH found in endocytic vesicles. J Biol Chem. 1984 Jul 25;259(14):9163–9171. [PubMed] [Google Scholar]
- Dimmock N. J. Review article initial stages in infection with animal viruses. J Gen Virol. 1982 Mar;59(Pt 1):1–22. doi: 10.1099/0022-1317-59-1-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Meador S. A., Levine A. S. Synthesis and processing of the precursor to the major core protein of adenovirus type 2. J Virol. 1977 Jan;21(1):199–214. doi: 10.1128/jvi.21.1.199-214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Philipson L. Mechanism of the arginine requirement for adenovirus synthesis. I. Synthesis of structural proteins. J Virol. 1971 Nov;8(5):742–753. doi: 10.1128/jvi.8.5.742-753.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald D. J., Padmanabhan R., Pastan I., Willingham M. C. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983 Feb;32(2):607–617. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- Fries E., Helenius A. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur J Biochem. 1979 Jun;97(1):213–220. doi: 10.1111/j.1432-1033.1979.tb13105.x. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Role of disulphide and sulphydryl groups in clustering of enkephalin receptors in neuroblastoma cells. Nature. 1979 Dec 6;282(5739):626–628. doi: 10.1038/282626a0. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- Kohn A. Early interactions of viruses with cellular membranes. Adv Virus Res. 1979;24:223–276. doi: 10.1016/s0065-3527(08)60395-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Wrigley N. G., Pereira H. G. Removal of pentons from particles of adenovirus type 2. Virology. 1969 Nov;39(3):599–604. doi: 10.1016/0042-6822(69)90111-1. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early events of virus-cell interaction in an adenovirus system. J Virol. 1969 Oct;4(4):323–338. doi: 10.1128/jvi.4.4.323-338.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Philipson L. Early interaction between animal viruses and cells. Monogr Virol. 1974;9:1–148. [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I. S., Steinman R. M., Unkeless J. C., Cohn Z. A. Selective iodination and polypeptide composition of pinocytic vesicles. J Cell Biol. 1980 Sep;86(3):712–722. doi: 10.1083/jcb.86.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C., Rosenkranz H. S., Mednis B. Structure and development of viruses as observed in the electron microscope. V. Entry and uncoating of adenovirus. J Virol. 1969 Nov;4(5):777–796. doi: 10.1128/jvi.4.5.777-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Persson R., Svensson U., Everitt E. Virus receptor interaction in the adenovirus system. II. Capping and cooperative binding of virions on HeLa cells. J Virol. 1983 Jun;46(3):956–963. doi: 10.1128/jvi.46.3.956-963.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. I. Purification and characterization of the adenovirus type 2 hexon antigen. Virology. 1967 Dec;33(4):575–590. doi: 10.1016/0042-6822(67)90057-8. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Philipson L., Höglund S. Structural proteins of adenoviruses. II. Purification and characterization of the adenovirus type 2 fiber antigen. Virology. 1968 Jun;35(2):204–215. doi: 10.1016/0042-6822(68)90261-4. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Ray B., Wu H. C. Internalization of ricin in Chinese hamster ovary cells. Mol Cell Biol. 1981 Jun;1(6):544–551. doi: 10.1128/mcb.1.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D. J., Willingham M. C., Pastan I. Role of a low-pH environment in adenovirus enhancement of the toxicity of a Pseudomonas exotoxin-epidermal growth factor conjugate. J Virol. 1984 Sep;51(3):650–655. doi: 10.1128/jvi.51.3.650-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Fitzgerald D., Ginsberg H., Willingham M., Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984 Aug;4(8):1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., Willingham M. C., Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984 Dec 10;259(23):14350–14353. [PubMed] [Google Scholar]
- Svensson U., Persson R. Entry of adenovirus 2 into HeLa cells. J Virol. 1984 Sep;51(3):687–694. doi: 10.1128/jvi.51.3.687-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson U., Persson R., Everitt E. Virus-receptor interaction in the adenovirus system I. Identification of virion attachment proteins of the HeLa cell plasma membrane. J Virol. 1981 Apr;38(1):70–81. doi: 10.1128/jvi.38.1.70-81.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Keith C. H., Maxfield F. R. Rapid acidification of endocytic vesicles containing asialoglycoprotein in cells of a human hepatoma line. J Cell Biol. 1983 Dec;97(6):1762–1776. doi: 10.1083/jcb.97.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- Weigel P. H., Oka J. A. Temperature dependence of endocytosis mediated by the asialoglycoprotein receptor in isolated rat hepatocytes. Evidence for two potentially rate-limiting steps. J Biol Chem. 1981 Mar 25;256(6):2615–2617. [PubMed] [Google Scholar]