Abstract
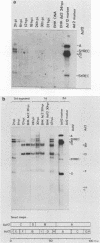
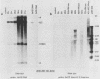
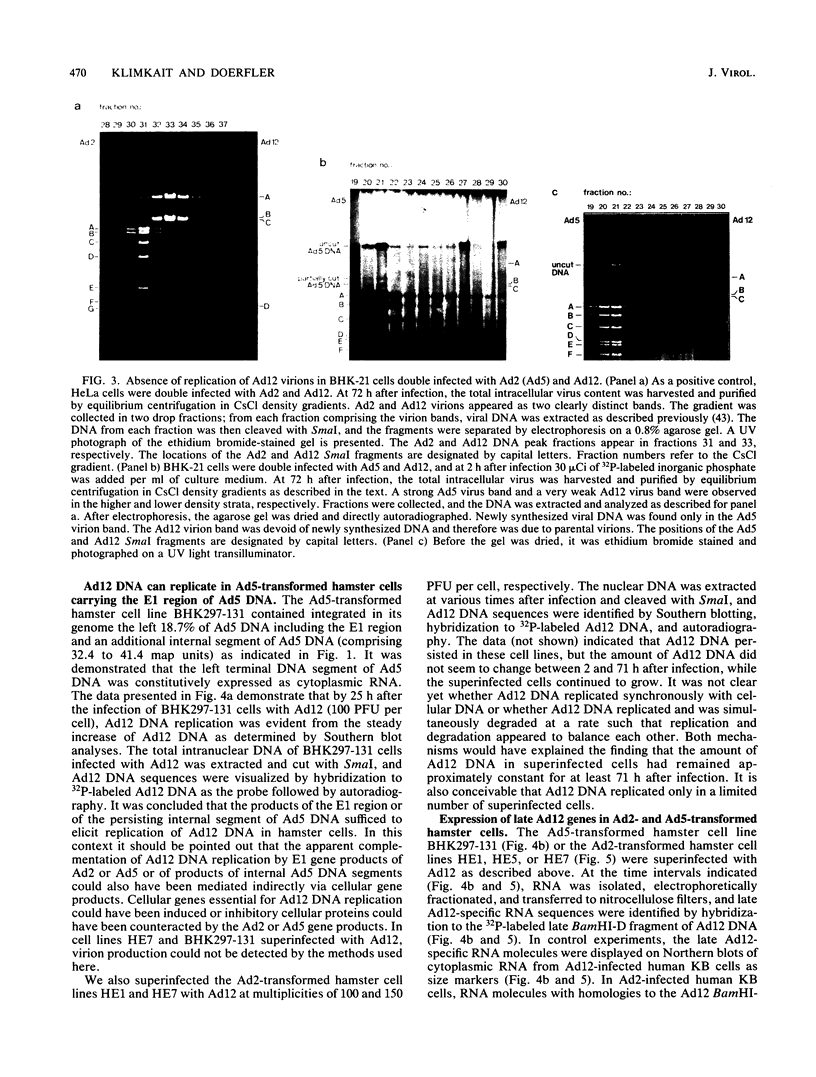
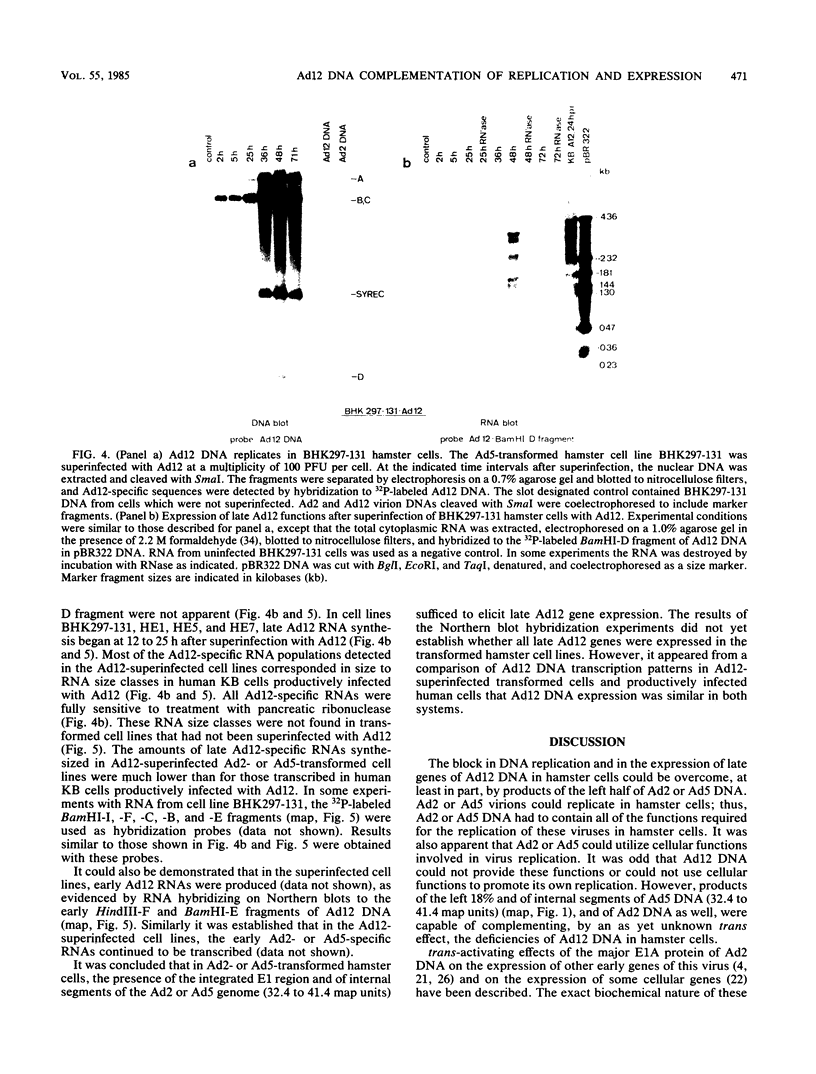
Human adenovirus type 12 (Ad12) cannot replicate in hamster cells. There is a complete block of viral DNA replication and of the expression of late viral genes. Early viral functions are expressed. In contrast, hamster cells are permissive for human adenovirus type 2 (Ad2). Some of the Ad12-specific functions are insufficient to support viral replication in hamster cells, or else cellular functions are missing or inhibitory for Ad12 replication. It was shown that the block in the replication and late expression of the Ad12 genome in hamster cells could, at least in part, be complemented by Ad2 and adenovirus type 5 (Ad5) functions. When hamster cells were coinfected with Ad2 (or Ad5) and Ad12, both Ad2 (Ad5) and Ad12 DNA replicated. Ad2 (Ad5) virions were produced in double-infected hamster cells. The assembly of intact Ad12 virions was not detectable by the techniques used here. The analysis was further refined by Ad12 superinfecting Ad2- or Ad5-transformed cells which carried in an integrated form defined fragments of the Ad2 or Ad5 genome. Persistence and continued expression of the left terminus of the Ad2 or Ad5 DNA in these cells has been documented and helped to support replication and late expression of Ad12 DNA. It remains to be determined which of the E1 functions of Ad2 or Ad5 were responsible for the helper effect. Investigations on the biochemical mechanism of this complementation will entail studies on very complex viral and possibly cellular functions involved in the control of viral gene expression.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Brüggemann U., Klenk H. D., Doerfler W. Increased infectivity of extracellular adenovirus type 12. J Virol. 1985 Jul;55(1):117–125. doi: 10.1128/jvi.55.1.117-125.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H., Doerfler W. Intracellular forms of adenovirus DNA. 3. Integration of the DNA of adenovirus type 2 into host DNA in productively infected cells. J Virol. 1974 May;13(5):975–992. doi: 10.1128/jvi.13.5.975-992.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Host response to adenovirus 2-transformed hamster embryo cells. Cancer Res. 1979 May;39(5):1455–1461. [PubMed] [Google Scholar]
- Deuring R., Klotz G., Doerfler W. An unusual symmetric recombinant between adenovirus type 12 DNA and human cell DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):3142–3146. doi: 10.1073/pnas.78.5.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Integration of the deoxyribonucleic acid of adenovirus type 12 into the deoxyribonucleic acid of baby hamster kidney cells. J Virol. 1970 Nov;6(5):652–666. doi: 10.1128/jvi.6.5.652-666.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U. Absence of replication of the DNA of adenovirus type 12 in BHK21 cells. Virology. 1970 Mar;40(3):754–757. doi: 10.1016/0042-6822(70)90222-9. [DOI] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Doerfler W., Stabel S., Ibelgaufts H., Sutter D., Neumann R., Groneberg J., Scheidtmann K. H., Deuring R., Winterhoff U. Selectivity in integration sites of adenoviral DNA. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):551–564. doi: 10.1101/sqb.1980.044.01.057. [DOI] [PubMed] [Google Scholar]
- Doerfler W. The fate of the DNA of adenovirus type 12 in baby hamster kidney cells. Proc Natl Acad Sci U S A. 1968 Jun;60(2):636–643. doi: 10.1073/pnas.60.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Schilling R., Doerfler W. In vitro translation of adenovirus type 12-specific mRNA isolated from infected and transformed cells. J Virol. 1979 Apr;30(1):21–31. doi: 10.1128/jvi.30.1.21-31.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esche H., Siegmann B. Expression of early viral gene products in adenovirus type 12-infected and -transformed cells. J Gen Virol. 1982 May;60(Pt 1):99–113. doi: 10.1099/0022-1317-60-1-99. [DOI] [PubMed] [Google Scholar]
- Fanning E., Doerfler W. Intracellular forms of adenovirus DNA. V. Viral DNA sequences in hamster cells abortively infected and transformed with human adenovirus type 12. J Virol. 1976 Nov;20(2):373–383. doi: 10.1128/jvi.20.2.373-383.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlmann R., Doerfler W. Integration of viral DNA into the genome of the adenovirus type 2-transformed hamster cell line HE5 without loss or alteration of cellular nucleotides. Nucleic Acids Res. 1983 Nov 11;11(21):7347–7361. doi: 10.1093/nar/11.21.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahlmann R., Leisten R., Vardimon L., Doerfler W. Patch homologies and the integration of adenovirus DNA in mammalian cells. EMBO J. 1982;1(9):1101–1104. doi: 10.1002/j.1460-2075.1982.tb01303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. T., Nevins J. R. Transcriptional activation and subsequent control of the human heat shock gene during adenovirus infection. Mol Cell Biol. 1983 Nov;3(11):2058–2065. doi: 10.1128/mcb.3.11.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruczek I., Doerfler W. The unmethylated state of the promoter/leader and 5'-regions of integrated adenovirus genes correlates with gene expression. EMBO J. 1982;1(4):409–414. doi: 10.1002/j.1460-2075.1982.tb01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Doerfler W. Mapping of Early and Late Transcripts Encoded by the Autographa californica Nuclear Polyhedrosis Virus Genome: Is Viral RNA Spliced? J Virol. 1984 May;50(2):497–506. doi: 10.1128/jvi.50.2.497-506.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H., Doerfler W. Transcription of overlapping sets of RNAs from the genome of Autographa californica nuclear polyhedrosis virus: a novel method for mapping RNAs. J Virol. 1984 Oct;52(1):255–265. doi: 10.1128/jvi.52.1.255-265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Ortin J., Doerfler W. Transcription of the genome of adenovirus type 12. I. Viral mRNA in abortively infected and transformed cells. J Virol. 1975 Jan;15(1):27–35. doi: 10.1128/jvi.15.1.27-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortin J., Scheidtmann K. H., Greenberg R., Westphal M., Doerfler W. Transcription of the genome of adenovirus type 12. III. Maps of stable RNA from productively infected human cells and abortively infected and transformed hamster cells. J Virol. 1976 Nov;20(2):355–372. doi: 10.1128/jvi.20.2.355-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPE J. H., ROWE W. P. IMMUNOFLUORESCENT STUDIES OF ADENOVIRUS 12 TUMORS AND OF CELLS TRANSFORMED OR INFECTED BY ADENOVIRUSES. J Exp Med. 1964 Oct 1;120:577–588. doi: 10.1084/jem.120.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raska K., Jr, Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. VI. Synthesis of virus-specific RNA. Virology. 1972 Mar;47(3):734–742. doi: 10.1016/0042-6822(72)90563-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rouse H. C., Strohl W. A., Schlesinger R. W. Properties of cells derived from adenovirus-induced hamster tumors by long-term in vitro cultivation. I. Clonal stability of three biological characteristics. Virology. 1966 Apr;28(4):633–644. doi: 10.1016/0042-6822(66)90248-0. [DOI] [PubMed] [Google Scholar]
- Rowe D. T., Graham F. L. Complementation of adenovirus type 5 host range mutants by adenovirus type 12 in coinfected HeLa and BHK-21 cells. J Virol. 1981 Apr;38(1):191–197. doi: 10.1128/jvi.38.1.191-197.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirm S., Doerfler W. Expression of viral DNA in adenovirus type 12-transformed cells, in tumor cells, and in revertants. J Virol. 1981 Sep;39(3):694–702. doi: 10.1128/jvi.39.3.694-702.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Shenk T., Williams J. Genetic analysis of adenoviruses. Curr Top Microbiol Immunol. 1984;111:1–39. doi: 10.1007/978-3-642-69549-0_1. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Saito I., Maruyama K., Shimojo H. Isolation of a nondefective recombinant between adenovirus type 5 and early region 1A of adenovirus type 12. J Virol. 1983 May;46(2):632–637. doi: 10.1128/jvi.46.2.632-637.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohl W. A., Rouse H., Teets K., Schlesinger R. W. The response of BHK21 cells to infection with type 12 adenovirus. 3. Transformation and restricted replication of superinfecting type 2 adenovirus. Arch Gesamte Virusforsch. 1970;31(1):93–112. doi: 10.1007/BF01241669. [DOI] [PubMed] [Google Scholar]
- Strohl W. A. The response of BHK21 cells to infection with type 12 adenovirus. II. Relationship of virus-stimulated DNA synthesis to other viral functions. Virology. 1969 Dec;39(4):653–665. doi: 10.1016/0042-6822(69)90004-x. [DOI] [PubMed] [Google Scholar]
- Sutter D., Westphal M., Doerfler W. Patterns of integration of viral DNA sequences in the genomes of adenovirus type 12-transformed hamster cells. Cell. 1978 Jul;14(3):569–585. doi: 10.1016/0092-8674(78)90243-x. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Doerfler W. Patterns of integration of viral DNA in adenovirus type 2-transformed hamster cells. J Mol Biol. 1981 Apr 5;147(2):227–246. doi: 10.1016/0022-2836(81)90439-3. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser L., van Maarschalkerweerd M. W., Rozijn T. H., Wassenaar A. D., Reemst A. M., Sussenbach J. S. Viral DNA sequences in adenovirus-transformed cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):541–550. doi: 10.1101/sqb.1980.044.01.056. [DOI] [PubMed] [Google Scholar]
- Vogel S., Brötz M., Kruczek I., Neumann R., Eick D., Winterhoff U., Doerfler W. Cloned fragments of human adenovirus type-12 DNA. Gene. 1981 Nov;15(2-3):273–278. doi: 10.1016/0378-1119(81)90136-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]