Abstract
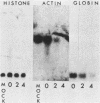
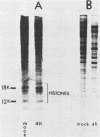
The consequences of herpes simplex virus type 1 infection on cellular macromolecules were investigated in Friend erythroleukemia cells. The patterns of protein synthesis, examined by polyacrylamide gel electrophoresis, demonstrated that by 4 h postinfection the synthesis of many host proteins, with the exception of histones, was inhibited. Examination of the steady-state level of histone H3 mRNA by molecular hybridization of total RNA to a cloned mouse histone H3 complementary DNA probe demonstrated that the ratio of histone H3 mRNA to total RNA remained unchanged for the first 4 h postinfection. In contrast, the steady-state levels of globin and actin mRNAs decreased progressively at early intervals postinfection. Studies on RNA synthesis in isolated nuclei demonstrated that the transcription of the histone H3 gene was inhibited to approximately the same extent as that of actin gene. We concluded that the stabilization of preexisting histone H3 mRNA was responsible for the persistence of H3 mRNA and histone protein synthesis in herpes simplex virus type 1-infected Friend erythroleukemia cells. The possible mechanisms influencing the differential stability of host mRNAs during the course of productive infection with herpes simplex virus type 1 are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURELIAN L., ROIZMAN B. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. II. ALTERNATIVE SUPPRESSION OF SYNTHESIS OF INTERFERON AND VIRAL CONSTITUENTS. J Mol Biol. 1965 Mar;11:539–548. doi: 10.1016/s0022-2836(65)80009-2. [DOI] [PubMed] [Google Scholar]
- AURELIAN L., ROIZMAN B. THE HOST RANGE OF HERPES SIMPLEX VIRUS; INTERFERON, VIRAL DNA, AND ANTIGEN SYNTHESIS IN ABORTIVE INFECTION OF DOG KIDNEY CELLS. Virology. 1964 Apr;22:452–461. doi: 10.1016/0042-6822(64)90066-2. [DOI] [PubMed] [Google Scholar]
- Adesnik M., Darnell J. E. Biogenesis and characterization of histone messenger RNA in HeLa cells. J Mol Biol. 1972 Jun 28;67(3):397–406. doi: 10.1016/0022-2836(72)90458-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. B., Mueller G. C. Control of histone synthesis in HeLa cells. Biochim Biophys Acta. 1973 Feb 4;294(1):481–496. doi: 10.1016/0005-2787(73)90104-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- DeLisle A. J., Graves R. A., Marzluff W. F., Johnson L. F. Regulation of histone mRNA production and stability in serum-stimulated mouse 3T6 fibroblasts. Mol Cell Biol. 1983 Nov;3(11):1920–1929. doi: 10.1128/mcb.3.11.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Flanagan J. F. Virus-specific ribonucleic acid synthesis in KB cells infected with herpes simplex virus. J Virol. 1967 Jun;1(3):583–590. doi: 10.1128/jvi.1.3.583-590.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallwitz D. Kinetics of inactivation of histone mRNA in the cytoplasm after inhibition of DNA replication in synchronised HeLa cells. Nature. 1975 Sep 18;257(5523):247–248. doi: 10.1038/257247a0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D., Mueller G. C. Histone synthesis in vitro on HeLa cell microsomes. The nature of the coupling to deoxyribonucleic acid synthesis. J Biol Chem. 1969 Nov 10;244(21):5947–5952. [PubMed] [Google Scholar]
- Greenberg J. R., Perry R. P. Relative occurrence of polyadenylic acid sequences in messenger and heterogeneous nuclear RNA of L cells as determined by poly (U)-hydroxylapatite chromatography. J Mol Biol. 1972 Dec 14;72(1):91–98. doi: 10.1016/0022-2836(72)90070-8. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I., Tanese N., Fuchs E. Complementary DNA sequence of a human cytoplasmic actin. Interspecies divergence of 3' non-coding regions. J Mol Biol. 1983 Feb 5;163(4):673–678. doi: 10.1016/0022-2836(83)90117-1. [DOI] [PubMed] [Google Scholar]
- Hay J., Koteles G. J., Keir H. M., Subak Sharpe H. Herpes virus specified ribonucleic acids. Nature. 1966 Apr 23;210(5034):387–390. doi: 10.1038/210387b0. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XI. Identification and relative molar rates of synthesis of structural and nonstructural herpes virus polypeptides in the infected cell. J Virol. 1973 Dec;12(6):1347–1365. doi: 10.1128/jvi.12.6.1347-1365.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquemont B., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus: characterization of viral high molecular weight nuclear RNA. J Gen Virol. 1975 Nov;29(2):155–165. doi: 10.1099/0022-1317-29-2-155. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Soreq H. The regulatory function of poly(A) and adjacent 3' sequences in translated RNA. Prog Nucleic Acid Res Mol Biol. 1982;27:53–83. doi: 10.1016/s0079-6603(08)60597-8. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: identification and comparison of adenovirus 2 encoded transcripts synthesized in vitro and vivo. J Mol Biol. 1979 Nov 25;135(1):171–197. doi: 10.1016/0022-2836(79)90346-2. [DOI] [PubMed] [Google Scholar]
- Maxson R., Cohn R., Kedes L., Mohun T. Expression and organization of histone genes. Annu Rev Genet. 1983;17:239–277. doi: 10.1146/annurev.ge.17.120183.001323. [DOI] [PubMed] [Google Scholar]
- Melli M., Spinelli G., Arnold E. Synthesis of histone messenger RNA of HeLa cells during the cell cycle. Cell. 1977 Sep;12(1):167–174. doi: 10.1016/0092-8674(77)90194-5. [DOI] [PubMed] [Google Scholar]
- Mory Y. Y., Gefter M. L. Synthesis of messenger RNA-like molecules in isolated myeloma nuclei. Nucleic Acids Res. 1977 Jun;4(6):1739–1757. doi: 10.1093/nar/4.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
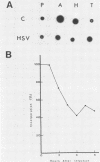
- Nakai H., Maxwell I. H., Pizer L. I. Herpesvirus infection alters the steady-state levels of cellular polyadenylated RNA in polyoma virus-transformed BHK cells. J Virol. 1982 Jun;42(3):1131–1134. doi: 10.1128/jvi.42.3.1131-1134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Jones G., Silverstein S. Inhibition by vesicular stomatitis virus of herpes simplex virus-directed protein synthesis. Virology. 1983 Jan 30;124(2):238–250. doi: 10.1016/0042-6822(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. The complete sequence of a chromosomal mouse alpha--globin gene reveals elements conserved throughout vertebrate evolution. Cell. 1979 Nov;18(3):875–882. doi: 10.1016/0092-8674(79)90139-9. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Alterations in the protein synthetic apparatus of Friend erythroleukemia cells infected with vesicular stomatitis virus or herpes simplex virus. J Virol. 1978 Jan;25(1):422–426. doi: 10.1128/jvi.25.1.422-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Beard P. The effect of herpes virus infection on mRNA in polyoma virus transformed cells. Virology. 1976 Dec;75(2):477–480. doi: 10.1016/0042-6822(76)90045-3. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Rice A. P., Roberts B. E. Vaccinia virus induces cellular mRNA degradation. J Virol. 1983 Sep;47(3):529–539. doi: 10.1128/jvi.47.3.529-539.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittman D. B., Graves R. A., Marzluff W. F. Histone mRNA concentrations are regulated at the level of transcription and mRNA degradation. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1849–1853. doi: 10.1073/pnas.80.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D., Pizer L. I. Herpesvirus infection modifies adenovirus RNA metabolism in adenovirus type 5-transformed cells. J Virol. 1978 Jul;27(1):1–12. doi: 10.1128/jvi.27.1.1-12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. The sedimentation profiles of cytoplasmic polyribosomes in mammalian cells productively and abortively infected with herpes simplex virus. Virology. 1968 Mar;34(3):562–565. doi: 10.1016/0042-6822(68)90075-5. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Sawicki S. G., White P. A., Darnell J. E., Jr A correlation between the rate of poly(A) shortening and half-life of messenger RNA in adenovirus transformed cells. J Mol Biol. 1978 Nov 25;126(1):23–36. doi: 10.1016/0022-2836(78)90277-2. [DOI] [PubMed] [Google Scholar]