Abstract
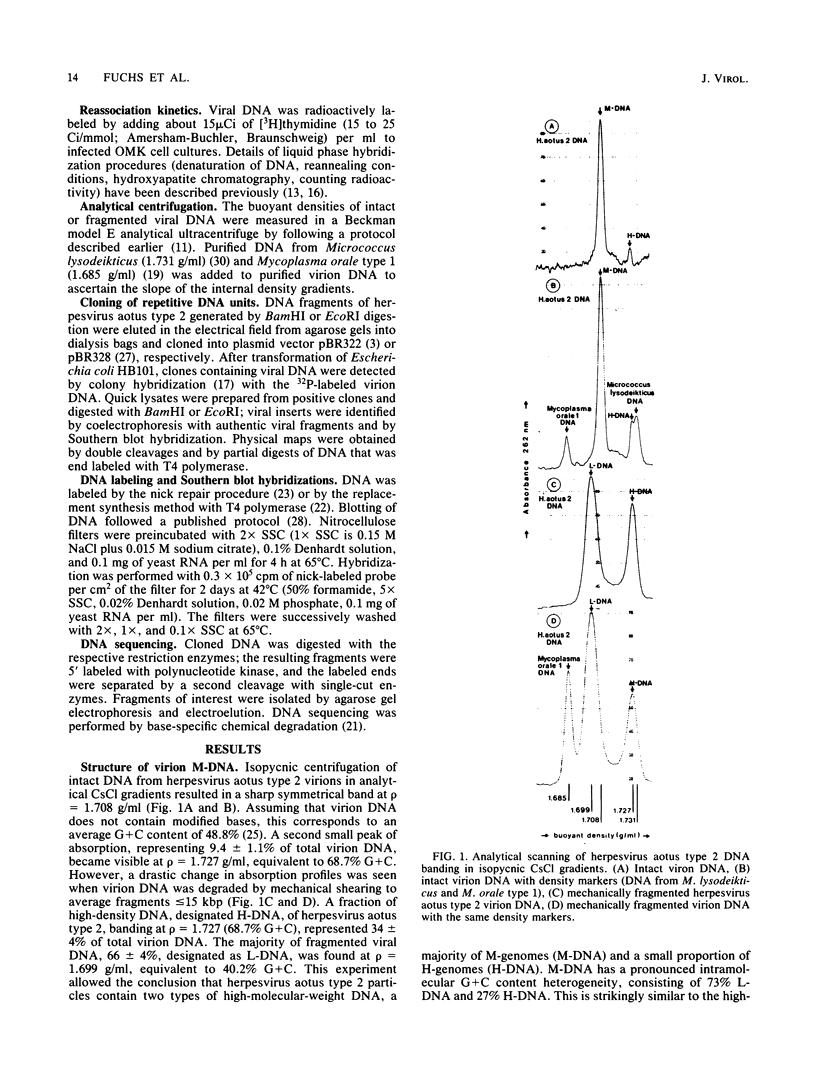
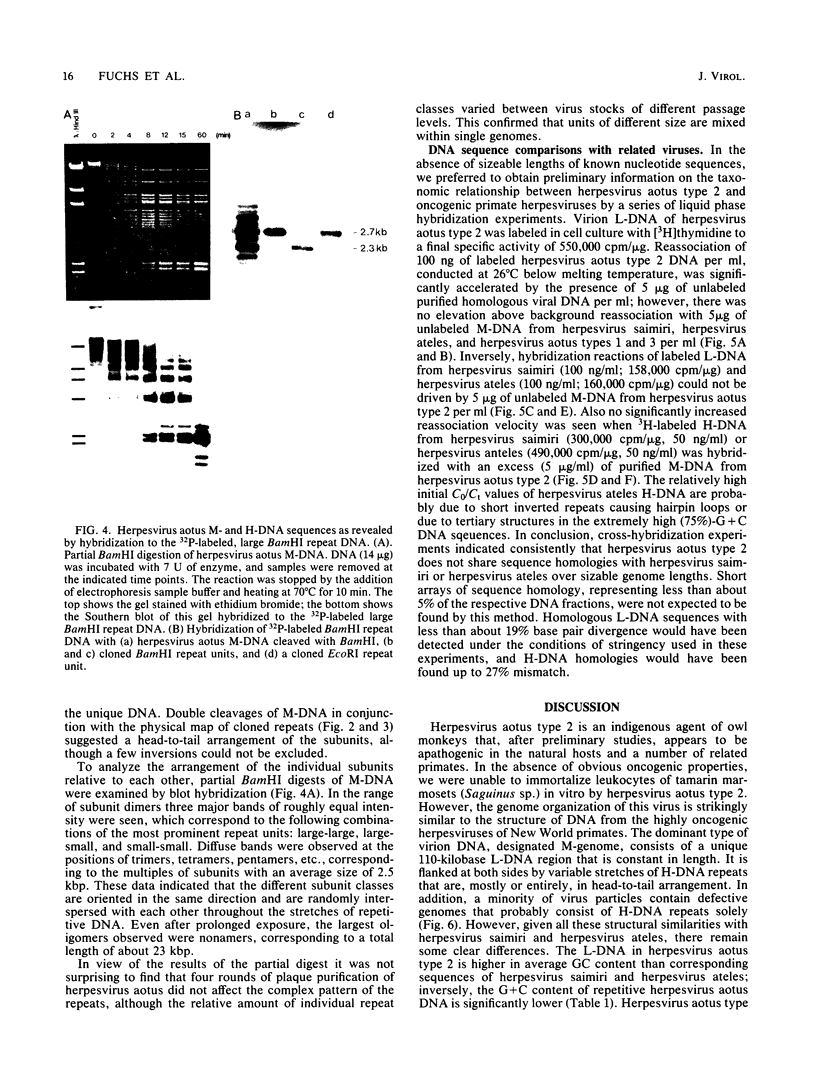
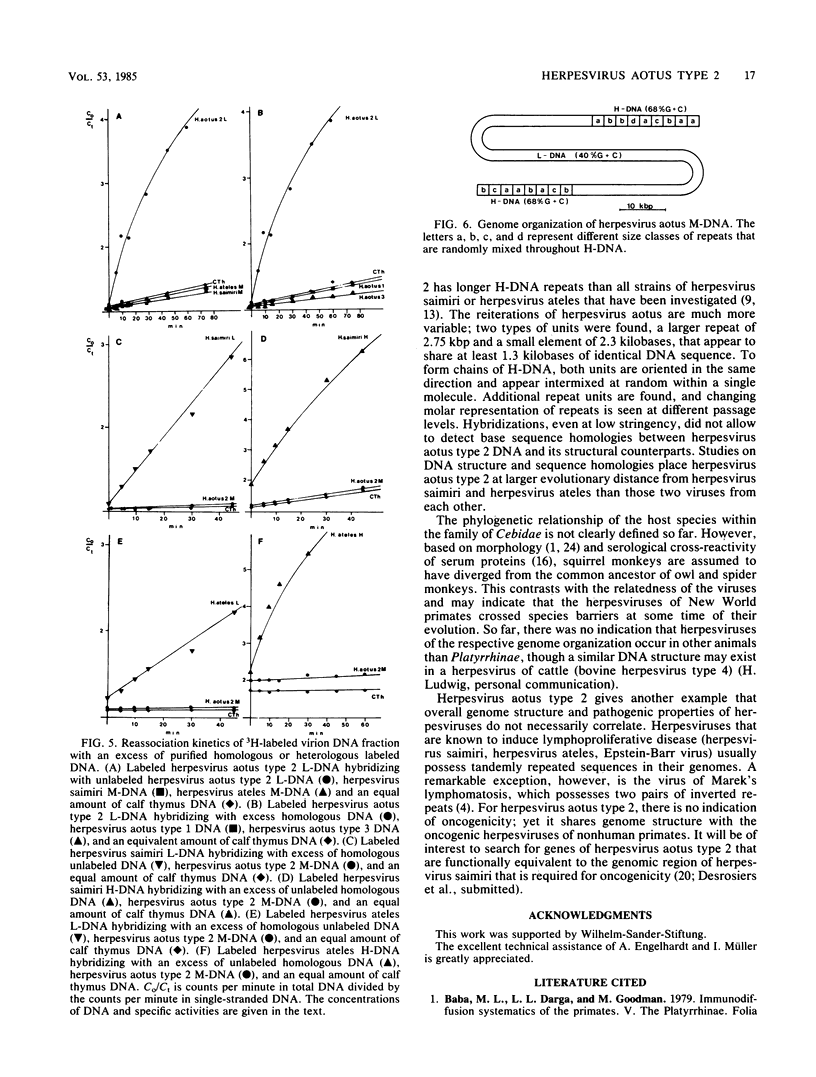
Herpesvirus aotus type 2, a virus commonly found in owl monkeys without overt disease, has a similar genome structure to the oncogenic herpesviruses of nonhuman primates (herpesvirus saimiri, herpesvirus ateles). Virion DNA of herpesvirus aotus type 2 (M-DNA) has an unique 110-kilobase-pair region of low G + C content (40.2%, L-DNA), inserted between stretches of repetitive H-DNA (68.7% G + C, about 41 kilobase pairs per molecule) that are variable in length. A minority of virions contain defective genomes that consist of repetitive H-DNA only. The H-DNA is composed of various types of repeat units that are related in sequence with each other. The two dominant types of repeats (2.3 and 2.7 kilobase pairs) were cloned and compared by restriction enzyme cleavages and partial nucleotide sequencing. They are homologous in at least 1.3 kilobase pairs. The two forms of repeat units are randomly arranged and oriented in tandem. Reassociation kinetics did not allow detection of sequence homologies between H- and L-DNA of herpesvirus aotus type 2 and the respective sequences of oncogenic primate herpesviruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barahona H. H., Meléndez L. V., King N. W., Daniel M. D., Fraser C. E., Preville A. C. Herpesvirus aotus type 2: a new viral agent from owl monkeys (Aotus trivirgatus). J Infect Dis. 1973 Feb;127(2):171–178. doi: 10.1093/infdis/127.2.171. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Cebrian J., Kaschka-Dierich C., Berthelot N., Sheldrick P. Inverted repeat nucleotide sequences in the genomes of Marek disease virus and the herpesvirus of the turkey. Proc Natl Acad Sci U S A. 1982 Jan;79(2):555–558. doi: 10.1073/pnas.79.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M. D., Melendez L. V., King N. W., Barahona H. H., Fraser C. E., Garcia F. G., Silva D. Isolation and characterization of a new virus from owl monkeys: herpesvirus aotus type 3. Am J Phys Anthropol. 1973 Mar;38(2):497–500. doi: 10.1002/ajpa.1330380254. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., King N. W., Fraser C. E., Barahona H. H., Hunt R. D., Garcia F. G., Trum B. F. Herpes virus aotus: a latent herpesvirus from owl monkeys (Aotus trivirgatus) isolation and characterization. Proc Soc Exp Biol Med. 1971 Dec;138(3):835–845. doi: 10.3181/00379727-138-36002. [DOI] [PubMed] [Google Scholar]
- Deinhardt F. W., Falk L. A., Wolfe L. G. Simian herpesviruses and neoplasia. Adv Cancer Res. 1974;19(0):167–205. doi: 10.1016/s0065-230x(08)60054-8. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Falk L. A. Herpesvirus saimiri strain variability. J Virol. 1982 Jul;43(1):352–356. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebeling A., Keil G., Nowak B., Fleckenstein B., Berthelot N., Sheldrick P. Genome structure and virion polypeptides of the primate herpesviruses Herpesvirus aotus types 1 and 3: comparison with human cytomegalovirus. J Virol. 1983 Feb;45(2):715–726. doi: 10.1128/jvi.45.2.715-726.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Bornkamm G. W., Mulder C., Werner F. J., Daniel M. D., Falk L. A., Delius H. Herpesvirus ateles DNA and its homology with Herpesvirus saimiri nucleic acid. J Virol. 1978 Jan;25(1):361–373. doi: 10.1128/jvi.25.1.361-373.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein B., Müller I., Werner J. The presence of Herpesvirus Saimiri genomes in virus-transformed cells. Int J Cancer. 1977 Apr 15;19(4):546–554. doi: 10.1002/ijc.2910190416. [DOI] [PubMed] [Google Scholar]
- Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979 Nov 30;560(3):301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Wallis J. Colony hybridization. Methods Enzymol. 1979;68:379–389. doi: 10.1016/0076-6879(79)68027-8. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton W. H., Mandel M. Deoxyribonucleic acid base compositions of mycoplasma strains of avian orgin. J Gen Microbiol. 1969 May;56(2):131–135. doi: 10.1099/00221287-56-2-131. [DOI] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tracy S., Desrosiers R. C. RNA from unique and repetitive DNA sequences of Herpesvirus saimiri. Virology. 1980 Jan 15;100(1):204–207. doi: 10.1016/0042-6822(80)90569-3. [DOI] [PubMed] [Google Scholar]
- Vanyushin B. F., Belozersky A. N., Kokurina N. A., Kadirova D. X. 5-methylcytosine and 6-methylamino-purine in bacterial DNA. Nature. 1968 Jun 15;218(5146):1066–1067. doi: 10.1038/2181066a0. [DOI] [PubMed] [Google Scholar]