Abstract
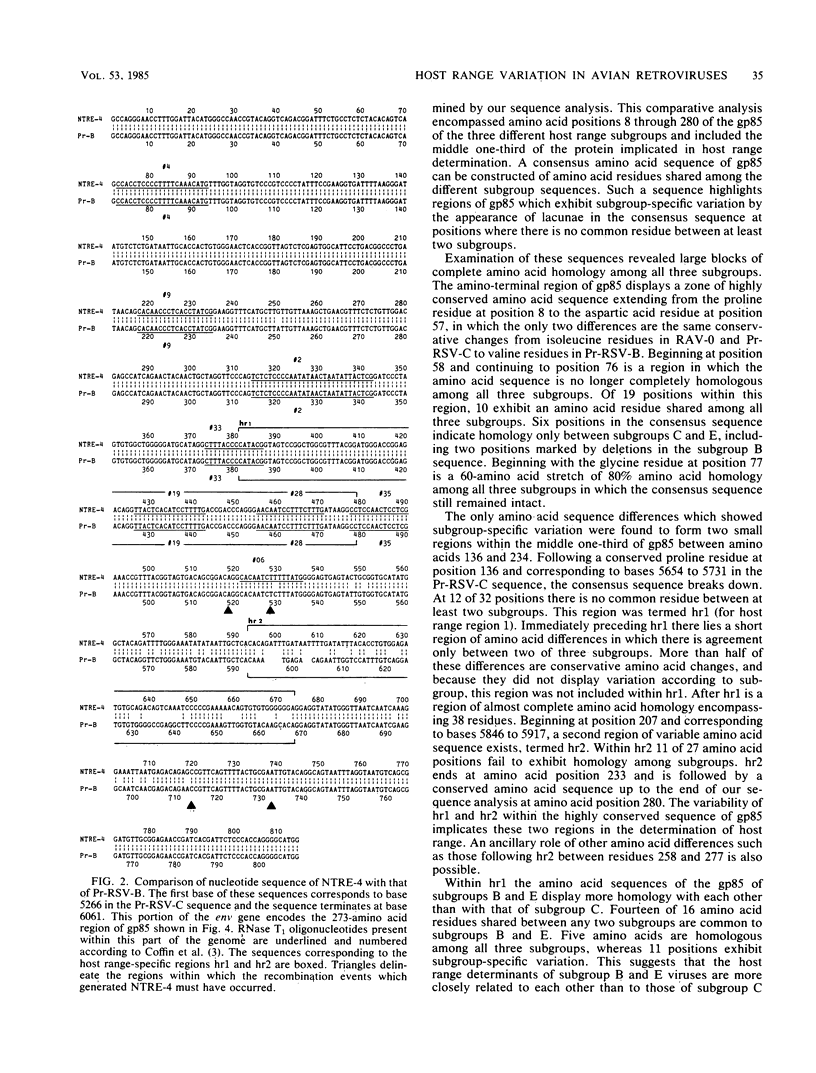
Previous genetic analysis has localized the region of the Rous sarcoma virus (RSV) env gene responsible for host range specificity to that encoding the middle one-third of gp85. To better understand the host range determinants, the relevant regions of the genomes of infectious molecular clones of the transformation-defective Prague strain of RSV, subgroup B (Pr-RSV-B) and Rous-associated virus 0 (RAV-0) (subgroup E) were sequenced and compared with the sequence of Pr-RSV-C. This comparative analysis identified two variable regions of low amino acid sequence homology flanked by highly conserved amino acid sequences. The first variable region (hr1) begins at base 5654 in the Pr-RSV-C sequence and encodes 32 amino acids. The second variable region (hr2) begins at base 5846 and encodes 27 amino acids. To test the role of the variable regions in host range specificity, we determined the sequence of this region of the env gene of NTRE-4, a recombinant virus between Pr-RSV-B and RAV-0 which exhibits an extended host range. This analysis revealed that the recombinant subgroup-encoding region of NTRE-4 is composed of 200 bases of RAV-0 sequence, including hr2, flanked by sequences which are otherwise of Pr-RSV-B origin. This study indicates that hr1 and hr2 are the domains of gp85 responsible for host range determination in avian retroviruses.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Tsichlis P. N., Barker C. S., Voynow S., Robinson H. L. Variation in avian retrovirus genomes. Ann N Y Acad Sci. 1980;354:410–425. doi: 10.1111/j.1749-6632.1980.tb27982.x. [DOI] [PubMed] [Google Scholar]
- Crittenden L. B., Motta J. V. The role of the tvb locus in genetic resistance to RSV(RAV-O). Virology. 1975 Oct;67(2):327–334. doi: 10.1016/0042-6822(75)90434-1. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Skalka A. M., Ju G. Endogenous avian retroviruses contain deficient promoter and leader sequences. Proc Natl Acad Sci U S A. 1983 May;80(10):2946–2950. doi: 10.1073/pnas.80.10.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R. G., Vogt P. K. Characteristics of two new avian tumor virus subgroups. Virology. 1969 Sep;39(1):18–30. doi: 10.1016/0042-6822(69)90344-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hunter E., Hill E., Hardwick M., Bhown A., Schwartz D. E., Tizard R. Complete sequence of the Rous sarcoma virus env gene: identification of structural and functional regions of its product. J Virol. 1983 Jun;46(3):920–936. doi: 10.1128/jvi.46.3.920-936.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R., Vogt P. K. Immunological relationships among envelope antigens of avian tumor viruses. Virology. 1966 Nov;30(3):375–387. doi: 10.1016/0042-6822(66)90116-4. [DOI] [PubMed] [Google Scholar]
- Joho R. H., Billeter M. A., Weissmann C. Mapping of biological functions on RNA of avian tumor viruses: location of regions required for transformation and determination of host range. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4772–4776. doi: 10.1073/pnas.72.12.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Omer C. A., Weis J. H., Mitsialis S. A., Faras A. J., Guntaka R. V. Restriction endonuclease and nucleotide sequence analyses of molecularly cloned unintegrated avian tumor virus DNA: structure of large terminal repeats in circle junctions. J Virol. 1982 Apr;42(1):346–351. doi: 10.1128/jvi.42.1.346-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moscovici C., Moscovici M. G., Jimenez H., Lai M. M., Hayman M. J., Vogt P. K. Continuous tissue culture cell lines derived from chemically induced tumors of Japanese quail. Cell. 1977 May;11(1):95–103. doi: 10.1016/0092-8674(77)90320-8. [DOI] [PubMed] [Google Scholar]
- Pani P. K. Further studies in genetic resistance of fowl to RSV(RAV O): evidence for interaction between independently segregating tumour virus b and tumour virus e genes. J Gen Virol. 1976 Sep;32(3):441–453. doi: 10.1099/0022-1317-32-3-441. [DOI] [PubMed] [Google Scholar]
- Payne L. N., Pani P. K. Ecidence for linkage between genetic loci controlling response of fowl to subgroup A and subgroup C sarcoma viruses. J Gen Virol. 1971 Nov;13(2):253–259. doi: 10.1099/0022-1317-13-2-253. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Bauer H., Graf T., Gelderblom H. Strain-specific antigen of the avian leukosis sarcoma virus group. I. Isolation and immunological characterization. Virology. 1970 Mar;40(3):530–539. doi: 10.1016/0042-6822(70)90196-0. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Conklin K. F., Coffin J. M. Mutant and recombinant avian retroviruses with extended host range. Proc Natl Acad Sci U S A. 1980 Jan;77(1):536–540. doi: 10.1073/pnas.77.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt P. K., Ishizaki R. Reciprocal patterns of genetic resistance to avian tumor viruses in two lines of chickens. Virology. 1965 Aug;26(4):664–672. doi: 10.1016/0042-6822(65)90329-6. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Joy A. E., Temin H. M. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J Virol. 1980 Jan;33(1):494–506. doi: 10.1128/jvi.33.1.494-506.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]


