Abstract
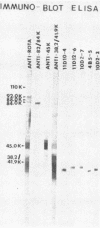
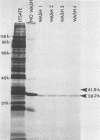
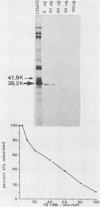
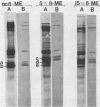
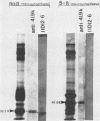
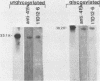
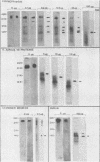
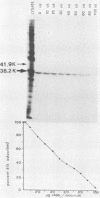
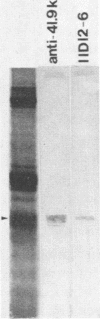
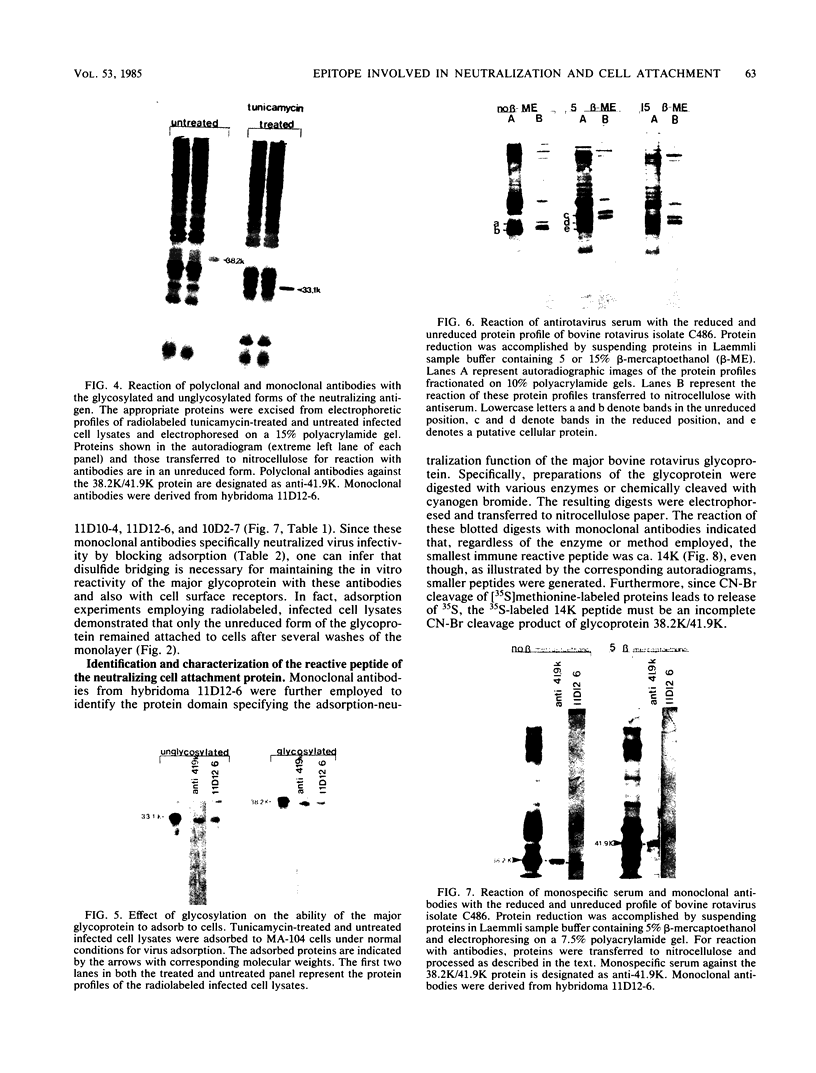
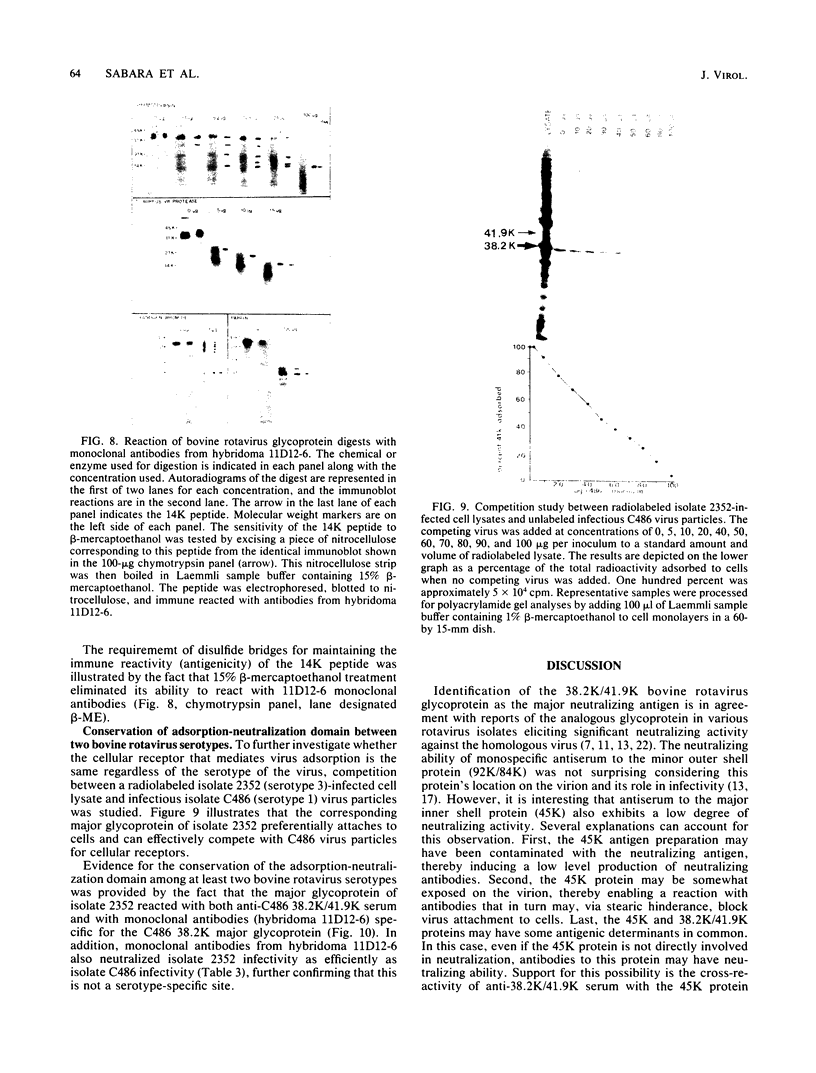
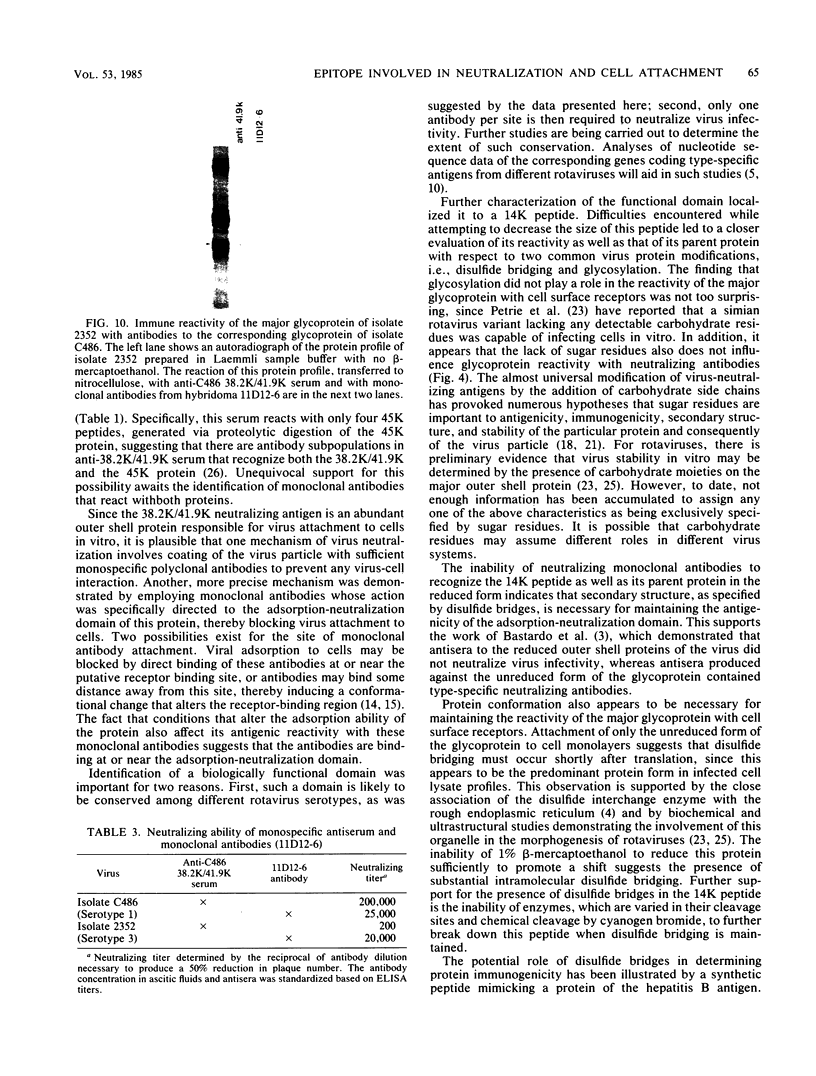
The 38,200-molecular weight (unreduced)/41,900-molecular-weight (reduced) glycoprotein of bovine rotavirus, isolate C486, was identified as the major neutralizing antigen. This glycoprotein as well as the corresponding glycoprotein of another bovine rotavirus serotype also specifically attached to cell monolayers under normal conditions for virus adsorption in vitro. Further support for this glycoprotein being directly responsible for virus attachment to cells was that (i) infectious virus of both serotypes could compete with the C486 glycoprotein for cell surface receptors, and (ii) neutralizing monospecific antiserum and neutralizing monoclonal antibodies directed toward the glycoprotein could block this virus-cell interaction. Preliminary epitope mapping of the glycoprotein with monoclonal antibodies further localized the neutralization-adsorption domain to a peptide with an approximate molecular weight of 14,000. The effect of two protein modifications, glycosylation and disulfide bridging, on the reactivity of this peptide with antibodies and cell surface receptors was investigated. It was demonstrated that, whereas glycosylation did not appear to affect these reactivities, disulfide bridging seemed to be essential.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon R., Shapira M., Jacob C. O. Synthetic vaccines. J Immunol Methods. 1983 Jul 29;61(3):261–273. doi: 10.1016/0022-1759(83)90220-x. [DOI] [PubMed] [Google Scholar]
- Babiuk L. A., Mohammed K., Spence L., Fauvel M., Petro R. Rotavirus isolation and cultivation in the presence of trypsin. J Clin Microbiol. 1977 Dec;6(6):610–617. doi: 10.1128/jcm.6.6.610-617.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Formation of intermolecular disulfide bonds on nascent immunoglobulin polypeptides. J Biol Chem. 1979 Jul 10;254(13):5690–5694. [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D. K., Pereira L., Norrild B., Roizman B. Application of denatured, electrophoretically separated, and immobilized lysates of herpes simplex virus-infected cells for detection of monoclonal antibodies and for studies of the properties of viral proteins. J Virol. 1983 Apr;46(1):103–112. doi: 10.1128/jvi.46.1.103-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dreesman G. R., Sanchez Y., Ionescu-Matiu I., Sparrow J. T., Six H. R., Peterson D. L., Hollinger F. B., Melnick J. L. Antibody to hepatitis B surface antigen after a single inoculation of uncoupled synthetic HBsAg peptides. Nature. 1982 Jan 14;295(5845):158–160. doi: 10.1038/295158a0. [DOI] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Graham D. Y., Ramig R. F., Ericson B. L. Heterogeneity in the structural glycoprotein (VP7) of simian rotavirus SA11. Virology. 1982 Oct 15;122(1):8–14. doi: 10.1016/0042-6822(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Flores J., Perez I., White L., Perez M., Kalica A. R., Marquina R., Wyatt R. G., Kapikian A. Z., Chanock R. M. Genetic relatedness among human rotaviruses as determined by RNA hybridization. Infect Immun. 1982 Aug;37(2):648–655. doi: 10.1128/iai.37.2.648-655.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F. X., Berger R., Tuma W., Kunz C. Location of immunodominant antigenic determinants on fragments of the tick-borne encephalitis virus glycoprotein: evidence for two different mechanisms by which antibodies mediate neutralization and hemagglutination inhibition. Virology. 1983 Oct 30;130(2):485–501. doi: 10.1016/0042-6822(83)90102-2. [DOI] [PubMed] [Google Scholar]
- Heinz F. X., Mandl C., Berger R., Tuma W., Kunz C. Antibody-induced conformational changes result in enhanced avidity of antibodies to different antigenic sites on the tick-borne encephalitis virus glycoprotein. Virology. 1984 Feb;133(1):25–34. doi: 10.1016/0042-6822(84)90422-7. [DOI] [PubMed] [Google Scholar]
- Infantile enteritis viruses: morphogenesis and morphology. J Virol. 1975 Oct;16(4):937–943. doi: 10.1128/jvi.16.4.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Keil W., Niemann H., Schwarz R. T., Klenk H. D. Carbohydrates of influenza virus. V. Oligosaccharides attached to individual glycosylation sites of the hemagglutinin of fowl plague virus. Virology. 1984 Feb;133(1):77–91. doi: 10.1016/0042-6822(84)90427-6. [DOI] [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leavitt R., Schlesinger S., Kornfeld S. Tunicamycin inhibits glycosylation and multiplication of Sindbis and vesicular stomatitis viruses. J Virol. 1977 Jan;21(1):375–385. doi: 10.1128/jvi.21.1.375-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Inouye S. Purification of an outer capsid glycoprotein of neonatal calf diarrhea virus and preparation of its antisera. Infect Immun. 1983 Jan;39(1):155–158. doi: 10.1128/iai.39.1.155-158.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
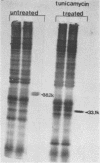
- Petrie B. L., Estes M. K., Graham D. Y. Effects of tunicamycin on rotavirus morphogenesis and infectivity. J Virol. 1983 Apr;46(1):270–274. doi: 10.1128/jvi.46.1.270-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabara M., Babiuk L. A., Gilchrist J., Misra V. Effect of tunicamycin on rotavirus assembly and infectivity. J Virol. 1982 Sep;43(3):1082–1090. doi: 10.1128/jvi.43.3.1082-1090.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voller A., Bidwell D. E. A simple method for detecting antibodies to rubella. Br J Exp Pathol. 1975 Aug;56(4):338–339. [PMC free article] [PubMed] [Google Scholar]