Abstract
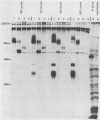
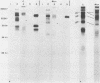
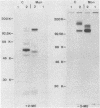
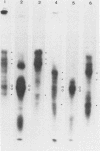
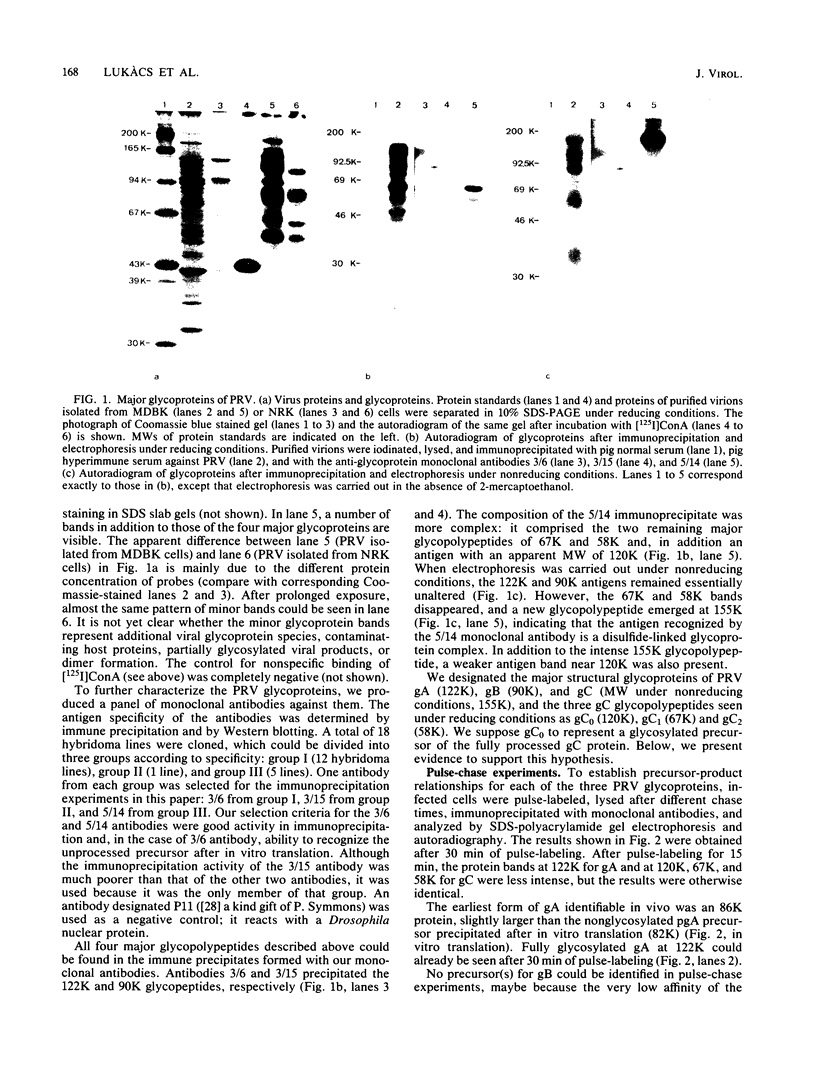
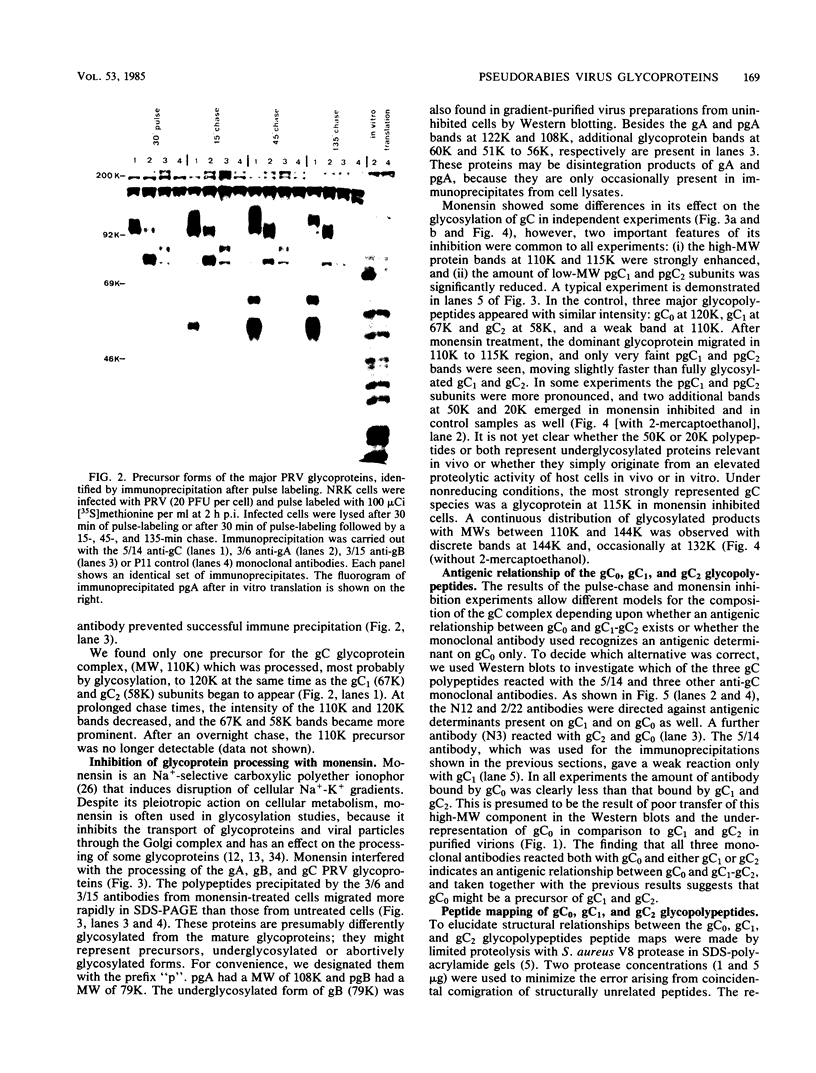
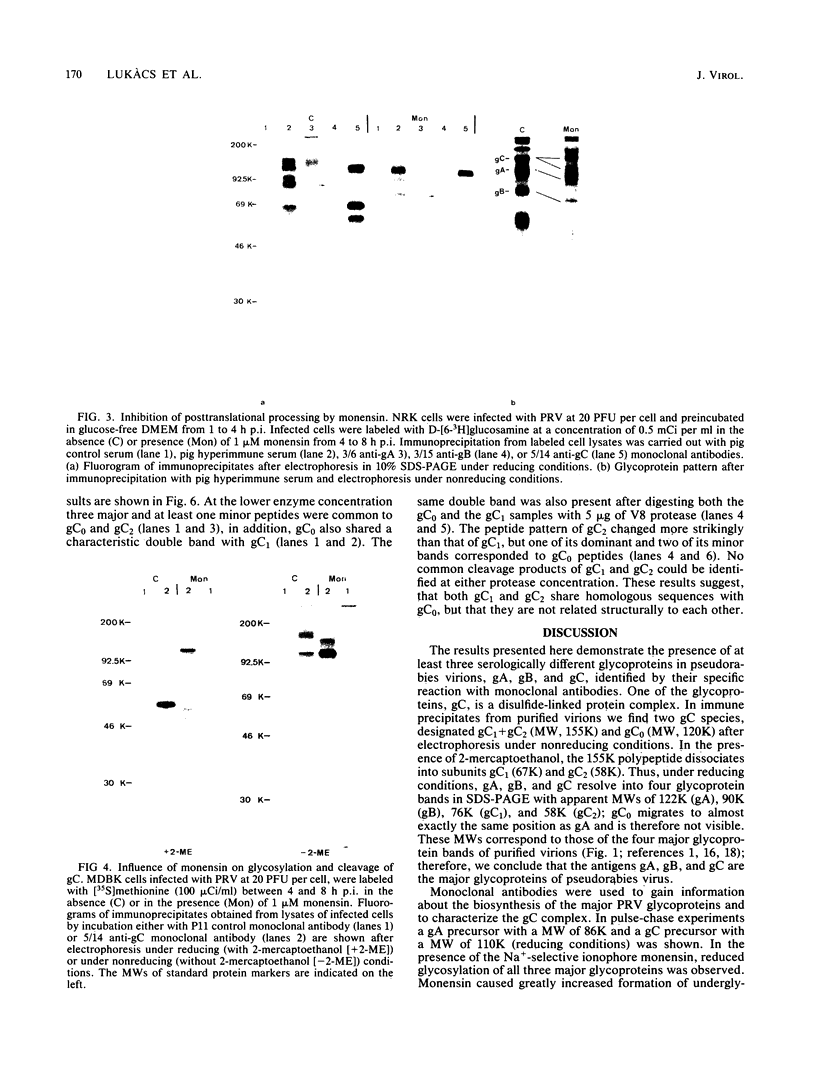
The glycoproteins of pseudorabies virus (PRV) Phylaxia were characterized with monoclonal antibodies as specific reagents. Three major structural glycoproteins with molecular weights of 155,000 (155K) (gC), 122K (gA), and 90K (gB) could be identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under nonreducing conditions. We investigated the processing of glycoproteins gA, gB, and gC by in vitro translation, pulse-chase experiments, and in the presence of the ionophore monensin which inhibits glycosylation. gA and gB were found to compose a single polypeptide, whereas gC was found to be a disulfide-linked glycoprotein complex. Immunoprecipitates formed with the aid of anti-gC monoclonal antibodies gave rise to three glycoprotein bands (gC0 [120K], gC1 [67K], and gC2 [58K]) by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions. Limited proteolysis of gC0, gC1, and gC2 resulted in peptide maps of gC0 related to those of both gC1 and gC2. No common peptide bands between gC1 and gC2, however, were seen. We suggest that (i) gC1 and gC2 arise by proteolytic cleavage from the same precursor molecule and stay joined via disulfide bridges and (ii) gC0 is an uncleaved precursor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Döller G., Flehmig B., Schmitz H. A comparison of enzyme-immunoassay and radioimmunoassay for detection of hepatitis A virus and antibodies against hepatitis A virus. J Biol Stand. 1984 Jan;12(1):47–59. doi: 10.1016/s0092-1157(84)80020-7. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Multimeric forms of herpes simplex virus type 2 glycoproteins. J Virol. 1982 Jan;41(1):348–351. doi: 10.1128/jvi.41.1.348-351.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein A. L., Jacquemont B., Machuca I. Infection of a restrictive cell line (XC cells) by intratypic recombinants of HSV-1: relationship between penetration of the virus and relative amounts of glycoprotein C. Virology. 1984 Jan 30;132(2):315–324. doi: 10.1016/0042-6822(84)90038-2. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose C., Edwards D. P., Weigle K. A., Friedrichs W. E., McGuire W. L. Varicella-zoster virus-specific gp140: a highly immunogenic and disulfide-linked structural glycoprotein. Virology. 1984 Jan 15;132(1):138–146. doi: 10.1016/0042-6822(84)90098-9. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr C. L., Pennington T. H. The effect of monensin on virion production and protein secretion in pseudorabies virus-infected cells. J Gen Virol. 1984 Jun;65(Pt 6):1033–1041. doi: 10.1099/0022-1317-65-6-1033. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Killington R. A., Yeo J., Honess R., Watson D. H., Duncan B. E., Halliburton I. W., Mumford J. Comparative analyses of the proteins and antigens of five herpesviruses. J Gen Virol. 1977 Nov;37(2):297–310. doi: 10.1099/0022-1317-37-2-297. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Ladin B. F., Ihara S., Hampl H., Ben-Porat T. Pathway of assembly of herpesvirus capsids: an analysis using DNA+ temperature-sensitive mutants of pseudorabies virus. Virology. 1982 Jan 30;116(2):544–561. doi: 10.1016/0042-6822(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lee G. T., Para M. F., Spear P. G. Location of the structural genes for glycoproteins gD and gE and for other polypeptides in the S component of herpes simplex virus type 1 DNA. J Virol. 1982 Jul;43(1):41–49. doi: 10.1128/jvi.43.1.41-49.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Spear P. G., Buchan A. Cell fusion induced by herpes simplex virus is promoted and suppressed by different viral glycoproteins. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3913–3917. doi: 10.1073/pnas.74.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukacs N., Rziha H. J. Mapping of the structural gene of pseudorabies virus glycoprotein A and identification of two non-glycosylated precursor polypeptides. J Virol. 1985 Jan;53(1):52–57. doi: 10.1128/jvi.53.1.52-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B. Immunochemistry of herpes simplex virus glycoproteins. Curr Top Microbiol Immunol. 1980;90:67–106. doi: 10.1007/978-3-642-67717-5_4. [DOI] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saumweber H., Symmons P., Kabisch R., Will H., Bonhoeffer F. Monoclonal antibodies against chromosomal proteins of Drosophila melanogaster: establishment of antibody producing cell lines and partial characterization of corresponding antigens. Chromosoma. 1980;80(3):253–275. doi: 10.1007/BF00292684. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Adams M. R., Rabin H. Glycosylation pathways of two major Epstein-Barr virus membrane antigens. Virology. 1983 May;127(1):168–176. doi: 10.1016/0042-6822(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]