Abstract
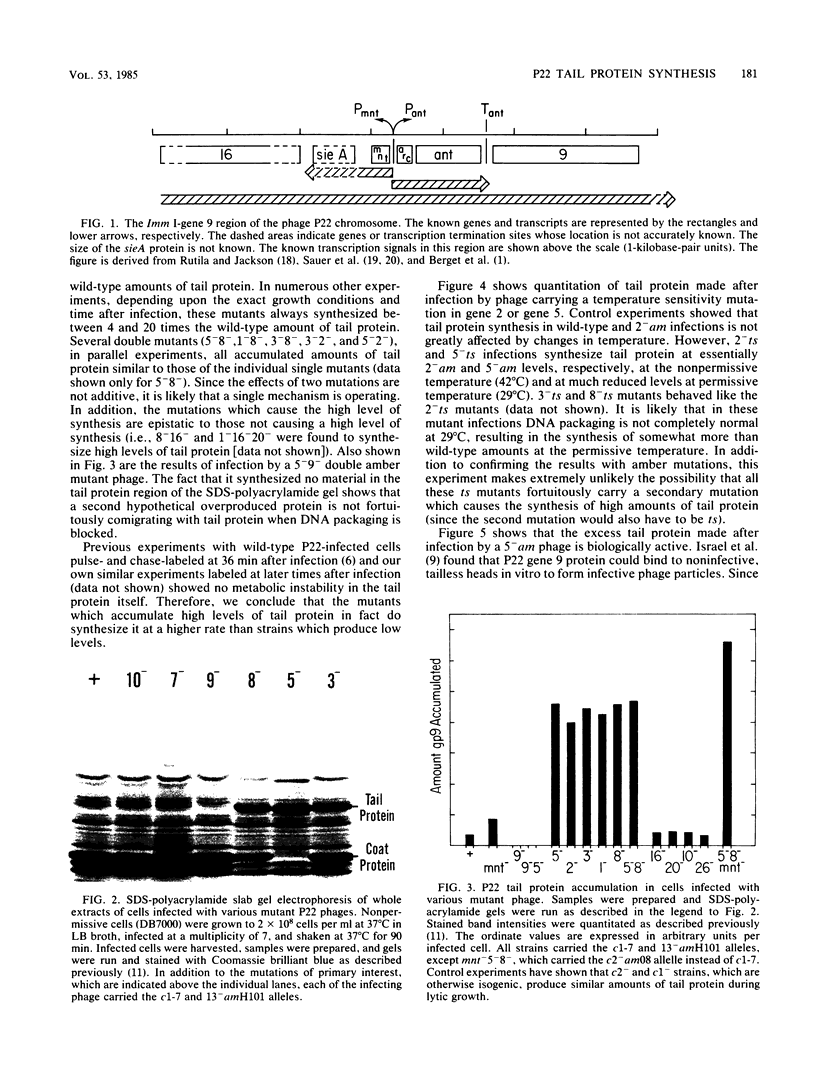
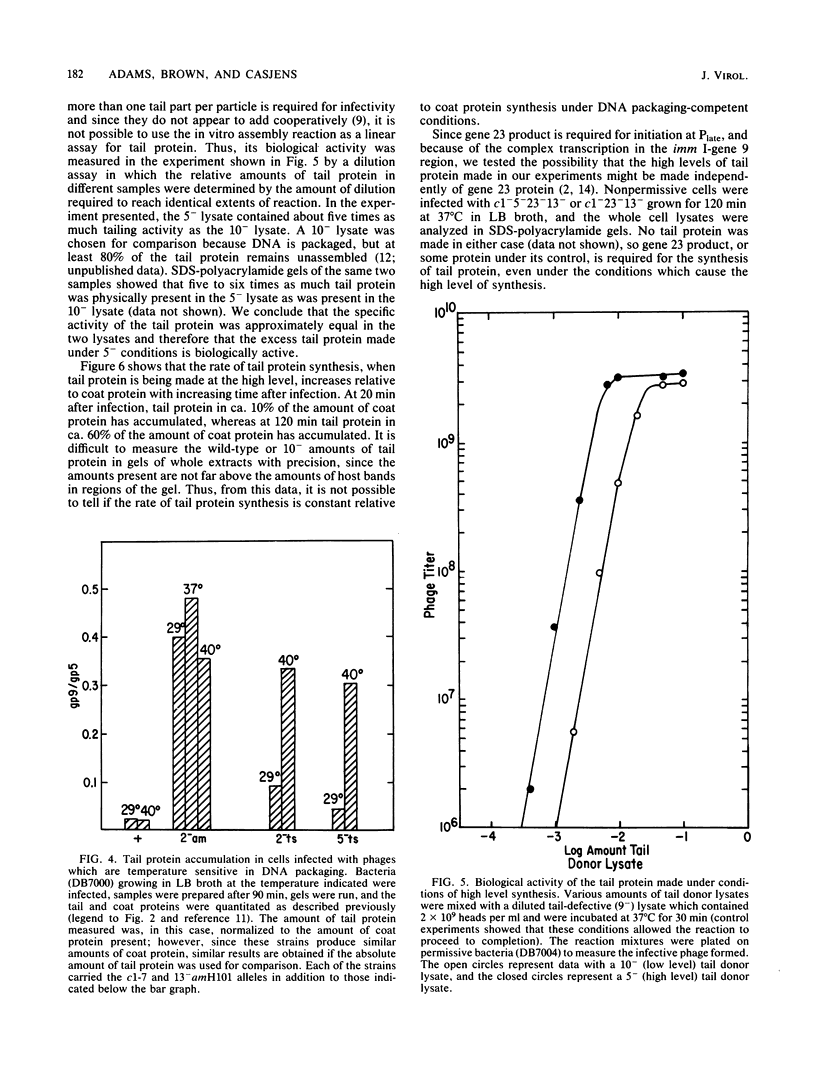
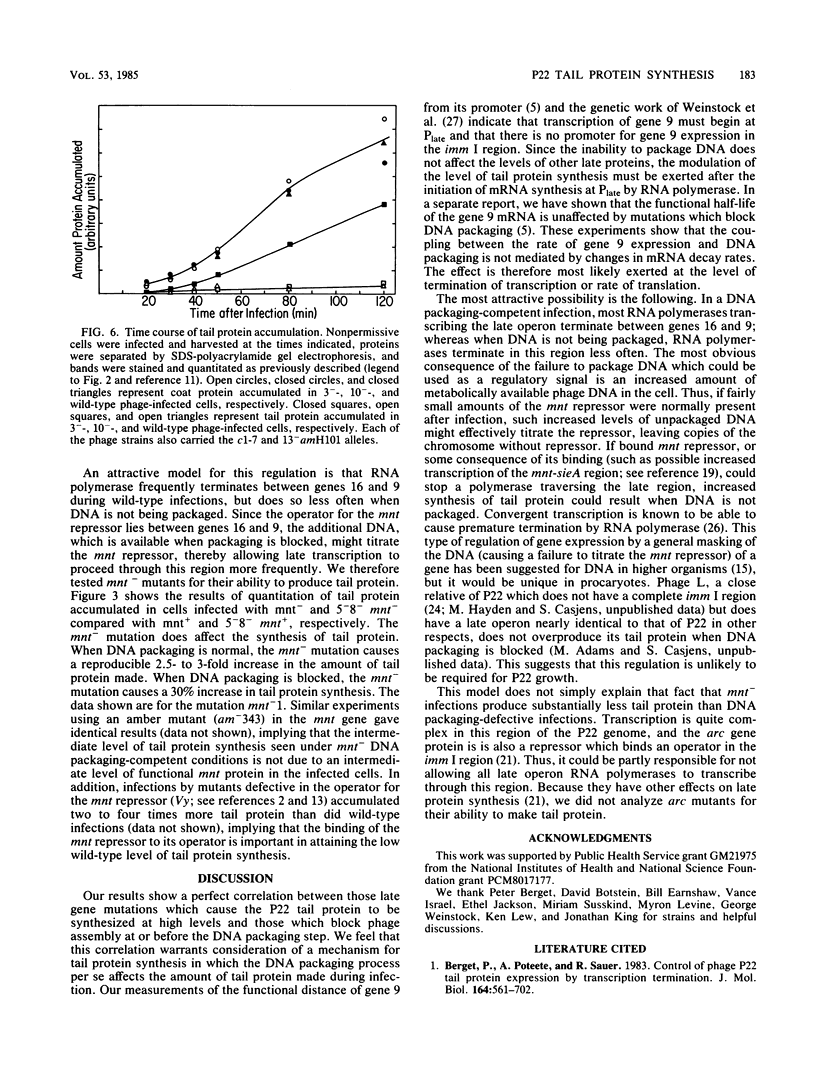
We have found that mutations which block bacteriophage P22 head assembly at or before the DNA packaging stage (1-, 2-, 3-, 5-, and 8-) cause up to a 20-fold increase in the amount of tail (gene 9) protein made during infection. This correlation seems strong enough to warrant consideration of a control mechanism in which the failure to package DNA per se causes a large increase in the synthesis of tail protein. Our results indicate that one of the repressors required for maintenance of lysogeny, the mnt gene product, may be partially responsible for this phenomenon.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget P. B., Poteete A. R., Sauer R. T. Control of phage P22 tail protein expression by transcription termination. J Mol Biol. 1983 Mar 15;164(4):561–572. doi: 10.1016/0022-2836(83)90050-5. [DOI] [PubMed] [Google Scholar]
- Botstein D., Waddell C. H., King J. Mechanism of head assembly and DNA encapsulation in Salmonella phage p22. I. Genes, proteins, structures and DNA maturation. J Mol Biol. 1973 Nov 15;80(4):669–695. doi: 10.1016/0022-2836(73)90204-0. [DOI] [PubMed] [Google Scholar]
- Botstein K., Lew K. K., Jarvik V., Swanson C. A. Role of antirepressor in the bipartite control of repression and immunity by bacteriophage P22. J Mol Biol. 1975 Feb 5;91(4):439–462. doi: 10.1016/0022-2836(75)90271-5. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Levine M. Virulent mutants of bacteriophage p22.I. Isolation and genetic analysis. J Virol. 1971 May;7(5):559–568. doi: 10.1128/jvi.7.5.559-568.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., Adams M. B. Posttranscriptional modulation of bacteriophage P22 scaffolding protein gene expression. J Virol. 1985 Jan;53(1):185–191. doi: 10.1128/jvi.53.1.185-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. 1. Exclusion of generalized transducing particles. Virology. 1971 Sep;45(3):629–637. doi: 10.1016/0042-6822(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Gough M. Establishment mode repressor synthesis blunts phage P22 antirepressor activity. J Mol Biol. 1977 Mar 25;111(1):55–64. doi: 10.1016/s0022-2836(77)80131-9. [DOI] [PubMed] [Google Scholar]
- Israel J. V., Anderson T. F., Levine M. in vitro MORPHOGENESIS OF PHAGE P22 FROM HEADS AND BASE-PLATE PARTS. Proc Natl Acad Sci U S A. 1967 Feb;57(2):284–291. doi: 10.1073/pnas.57.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvik J., Botstein D. Conditional-lethal mutations that suppress genetic defects in morphogenesis by altering structural proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2738–2742. doi: 10.1073/pnas.72.7.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Hall C., Casjens S. Control of the synthesis of phage P22 scaffolding protein is coupled to capsid assembly. Cell. 1978 Oct;15(2):551–560. doi: 10.1016/0092-8674(78)90023-5. [DOI] [PubMed] [Google Scholar]
- King J., Lenk E. V., Botstein D. Mechanism of head assembly and DNA encapsulation in Salmonella phage P22. II. Morphogenetic pathway. J Mol Biol. 1973 Nov 15;80(4):697–731. doi: 10.1016/0022-2836(73)90205-2. [DOI] [PubMed] [Google Scholar]
- Levine M., Truesdell S., Ramakrishnan T., Bronson M. J. Dual control of lysogeny by bacteriophage P22: an antirepressor locus and its controlling elements. J Mol Biol. 1975 Feb 5;91(4):421–438. doi: 10.1016/0022-2836(75)90270-3. [DOI] [PubMed] [Google Scholar]
- Lew K., Casjens S. Identification of early proteins coded by bacteriophage P22. Virology. 1975 Dec;68(2):525–533. doi: 10.1016/0042-6822(75)90292-5. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Pardon J. F. Structure and function of chromatin. Annu Rev Genet. 1979;13:197–233. doi: 10.1146/annurev.ge.13.120179.001213. [DOI] [PubMed] [Google Scholar]
- Poteete A. R., King J. Functions of two new genes in Salmonella phage P22 assembly. Virology. 1977 Feb;76(2):725–739. doi: 10.1016/0042-6822(77)90254-9. [DOI] [PubMed] [Google Scholar]
- Rutila J. E., Jackson E. N. Physical map of the bacteriophage P22 genome. Virology. 1981 Sep;113(2):769–775. doi: 10.1016/0042-6822(81)90206-3. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Krovatin W., DeAnda J., Youderian P., Susskind M. M. Primary structure of the immI immunity region of bacteriophage P22. J Mol Biol. 1983 Aug 25;168(4):699–713. doi: 10.1016/s0022-2836(83)80070-9. [DOI] [PubMed] [Google Scholar]
- Sauer R. T., Krovatin W., Poteete A. R., Berget P. B. Phage P22 tail protein: gene and amino acid sequence. Biochemistry. 1982 Nov 9;21(23):5811–5815. doi: 10.1021/bi00266a014. [DOI] [PubMed] [Google Scholar]
- Susskind M. M. A new gene of bacteriophage P22 which regulates synthesis of antirepressor. J Mol Biol. 1980 Apr 25;138(4):685–713. doi: 10.1016/0022-2836(80)90060-1. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Mechanism of action of Salmonella phage P22 antirepressor. J Mol Biol. 1975 Oct 25;98(2):413–424. doi: 10.1016/s0022-2836(75)80127-6. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978 Jun;42(2):385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Repression and immunity in Salmonella phages P22 and L: phage L lacks a functional secondary immunity system. Virology. 1978 Sep;89(2):618–622. doi: 10.1016/0042-6822(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D., Wright A. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. III. Failure of superinfecting phage DNA to enter sieA+ lysogens. Virology. 1974 Dec;62(2):350–366. doi: 10.1016/0042-6822(74)90398-5. [DOI] [PubMed] [Google Scholar]
- Ward D. F., Murray N. E. Convergent transcription in bacteriophage lambda: interference with gene expression. J Mol Biol. 1979 Sep 15;133(2):249–266. doi: 10.1016/0022-2836(79)90533-3. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., Riggs P. D., Botstein D. Genetics of bacteriophage P22. III. The late operon. Virology. 1980 Oct 15;106(1):82–91. doi: 10.1016/0042-6822(80)90223-8. [DOI] [PubMed] [Google Scholar]
- Winston F., Botstein D., Miller J. H. Characterization of amber and ochre suppressors in Salmonella typhimurium. J Bacteriol. 1979 Jan;137(1):433–439. doi: 10.1128/jb.137.1.433-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D. Lysogenization and superinfection immunity in Salmonella. Virology. 1958 Apr;5(2):291–326. doi: 10.1016/0042-6822(58)90025-4. [DOI] [PubMed] [Google Scholar]