Abstract
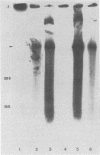
Ten stable temperature-sensitive mutants of Japanese encephalitis virus were isolated after mutagenesis by growth of cloned wild-type virus in the presence of the nucleic acid precursor analogs 5-fluorouracil and 5-azacytidine. Mutants were selected which grew at least 100-fold better at 33 degrees C than at 41 degrees C. The 5-fluorouracil was found to be more effective at inducing temperature-sensitive mutations than was 5-azacytidine. Analysis of the virus-specific RNA and proteins synthesized by each mutant at the nonpermissive temperature was used to determine biochemical phenotypes. The mutants were analyzed for abilities to complement in mixed infections. Although inefficient and sometimes nonreciprocal, complementation occurred at higher levels than previously reported for flavivirus mutants. Interference between mutants in some mixed infections was also observed. Seven complementation groups were defined. Three groups contained mutants incapable of synthesizing virus-specific RNA at the nonpermissive temperature, whereas the remaining complementation groups displayed an RNA+ phenotype. Levels of protein synthesis comparable to that of wild type were observed at the nonpermissive temperature in three groups. Two other groups were represented by mutants which synthesized only low levels of virus-specific proteins at the higher temperature. Mutants in the remaining two groups did not produce detectable levels of proteins under nonpermissive conditions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Altered E2 glycoprotein of Sindbis virus and its use in complementation studies. J Virol. 1978 Apr;26(1):126–135. doi: 10.1128/jvi.26.1.126-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Eckels K. H., Brandt W. E., Harrison V. R., McCown J. M., Russell P. K. Isolation of a temperature-sensitive dengue-2 virus under conditions suitable for vaccine development. Infect Immun. 1976 Nov;14(5):1221–1227. doi: 10.1128/iai.14.5.1221-1227.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle S., Zebovitz E. A spontaneous temperature sensitive mutant of Japanese encephalitis virus: preliminary characterization. Arch Virol. 1977;54(3):165–176. doi: 10.1007/BF01314783. [DOI] [PubMed] [Google Scholar]
- Hollingshead P. G., Jr, Brawner T. A., Fleming T. P. St. Louis encephalitis virus temperature-sensitive mutants. I. Induction, isolation and preliminary characterization. Arch Virol. 1983;75(3):171–179. doi: 10.1007/BF01315271. [DOI] [PubMed] [Google Scholar]
- Inoue Y. K., Ogura R. Studies on Japanese B encephalitis virus. III. Propagation and assay of Japanese B encephalitis virus in a stable line of porcine kidney cells. Virology. 1962 Feb;16:205–207. doi: 10.1016/0042-6822(62)90299-4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda S., Hashimoto K., Simizu B. Complementation between temperature-sensitive mutants isolated from Aedes albopictus cells persistently infected with Western equine encephalitis virus. Virology. 1979 Jan 30;92(2):532–541. doi: 10.1016/0042-6822(79)90155-7. [DOI] [PubMed] [Google Scholar]
- Pringle C. R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J Virol. 1970 May;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Lenches E. M., Strauss J. H. Mutants of sindbis virus. I. Isolation and partial characterization of 89 new temperature-sensitive mutants. Virology. 1976 Oct 1;74(1):154–168. doi: 10.1016/0042-6822(76)90137-9. [DOI] [PubMed] [Google Scholar]
- Tarr G. C., Lubiniecki A. S. Chemically induced temperature-sensitive mutants of dengue virus type 2: comparison of temperature sensitivity in vitro with infectivity suckling mice, hamsters, and rhesus monkeys. Infect Immun. 1976 Mar;13(3):688–695. doi: 10.1128/iai.13.3.688-695.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. C., Lubiniecki A. S. Chemically-induced temperature sensitive mutants of dengue virus type 2. I. Isolation and partial characterization. Arch Virol. 1976;50(3):223–235. doi: 10.1007/BF01320576. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weil P. A., Hampel A. Preparative agarose gel electrophoresis of ribonucleic acid. Biochemistry. 1973 Oct 23;12(22):4361–4367. doi: 10.1021/bi00746a010. [DOI] [PubMed] [Google Scholar]