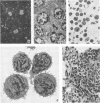
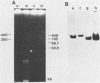
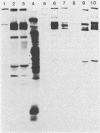
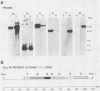
Abstract
A nonproducer lymphoblastoid cell line (7710) containing the herpesvirus saimiri (HVS) genome was established from the HVS-positive spleen of a male, inbred New Zealand White rabbit (III/J strain) which had developed a well-differentiated lymphoma after inoculation of HVS and 12-O-tetradecanoylphorbol-13-acetate (TPA). Antibodies to HVS early and late antigens were detected in the serum of rabbit 7710 by indirect immunofluorescence and immunoprecipitation. The cell line was of T-cell origin, did not produce HVS, and could not be superinfected with HVS. However, HVS early antigens could be induced in the cells with n-butyric acid and TPA or TPA alone. On the other hand, late antigens were never observed, and infectious virus could not be rescued by cocultivation of 7710 cell with OMK cells. The 7710 cells were T-cell growth factor dependent, even after many in vitro passages. The 7710 cell line contained multiple copies of a nonintegrated, covalently closed circular HVS genome. As is characteristic of some other HVS-transformed nonproducer lymphoid cell lines, a large segment of unique light (L) DNA was missing in the persistent circular viral DNA present in 7710 cells. This deletion spanned at least 42.5 kilobases, corresponding to the segment between 12.3 and 50.7 map units of full-length, infectious virion L-DNA. The 7710 cells failed to induce tumors in athymic nude mice, but inbred rabbits inoculated with as few as 100 of these cells developed fatal lymphomas. Chromosomal analysis showed that tumors were due to the growth of donor cells. Cells recovered from one of the rabbits inoculated with 7710 cells also contained HVS DNA and, after in vitro culture, induced the same type of lymphoma when inoculated into two other III/J-strain rabbits. None of the previously described HVS-transformed cell lines have been able to induce tumors in either their host species or nude mice. Thus, our demonstration that the 7710 cell line is readily transplantable in syngeneic rabbits represents the first available model which allows analysis of many biological and molecular aspects of the in vivo oncogenicity of HVS.
Full text
PDF


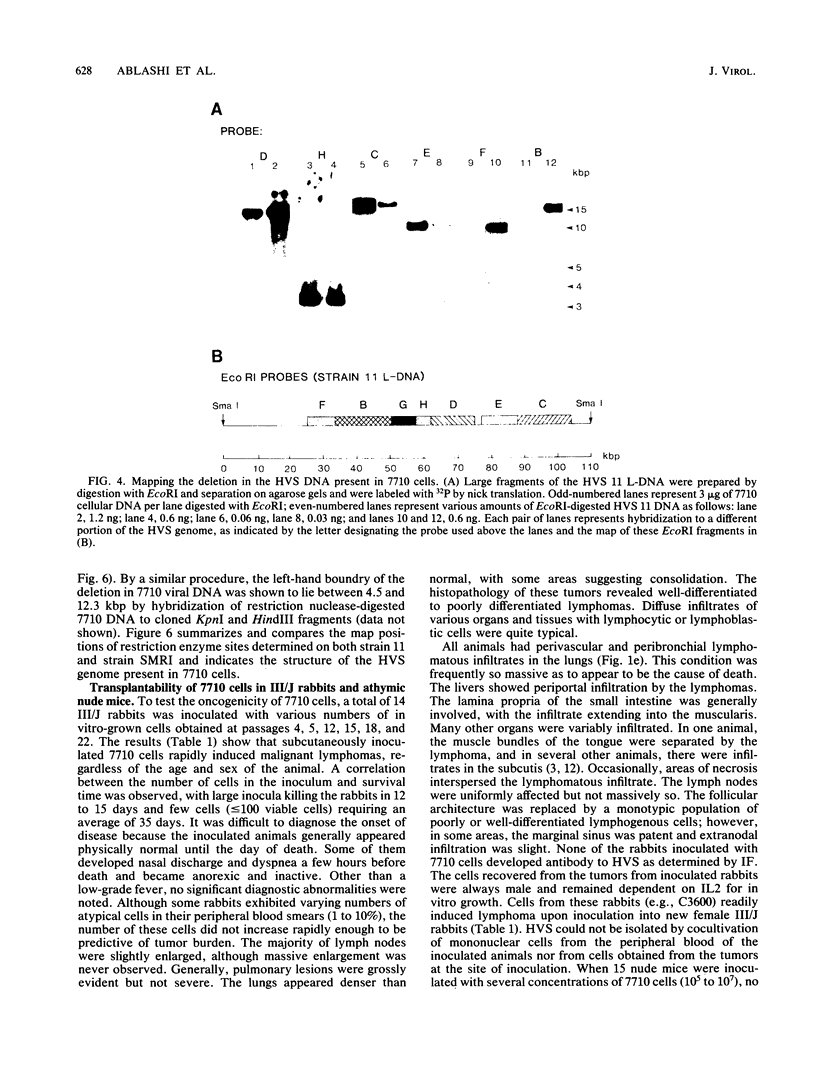

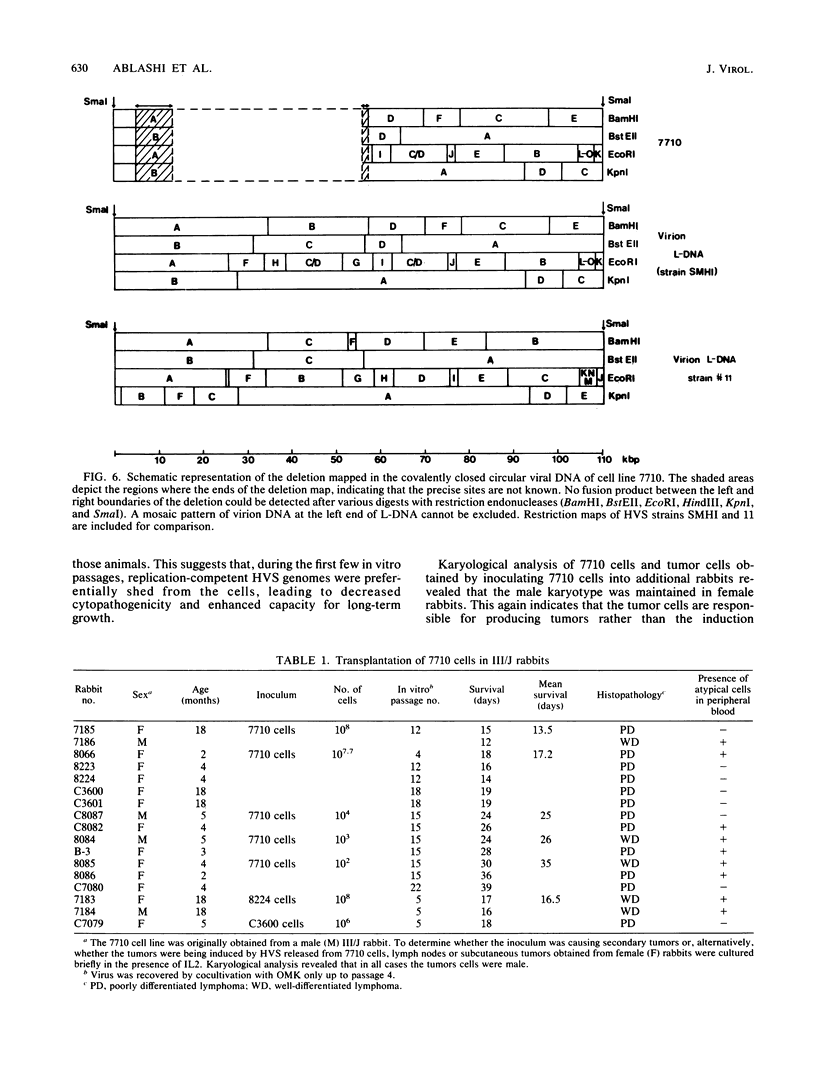
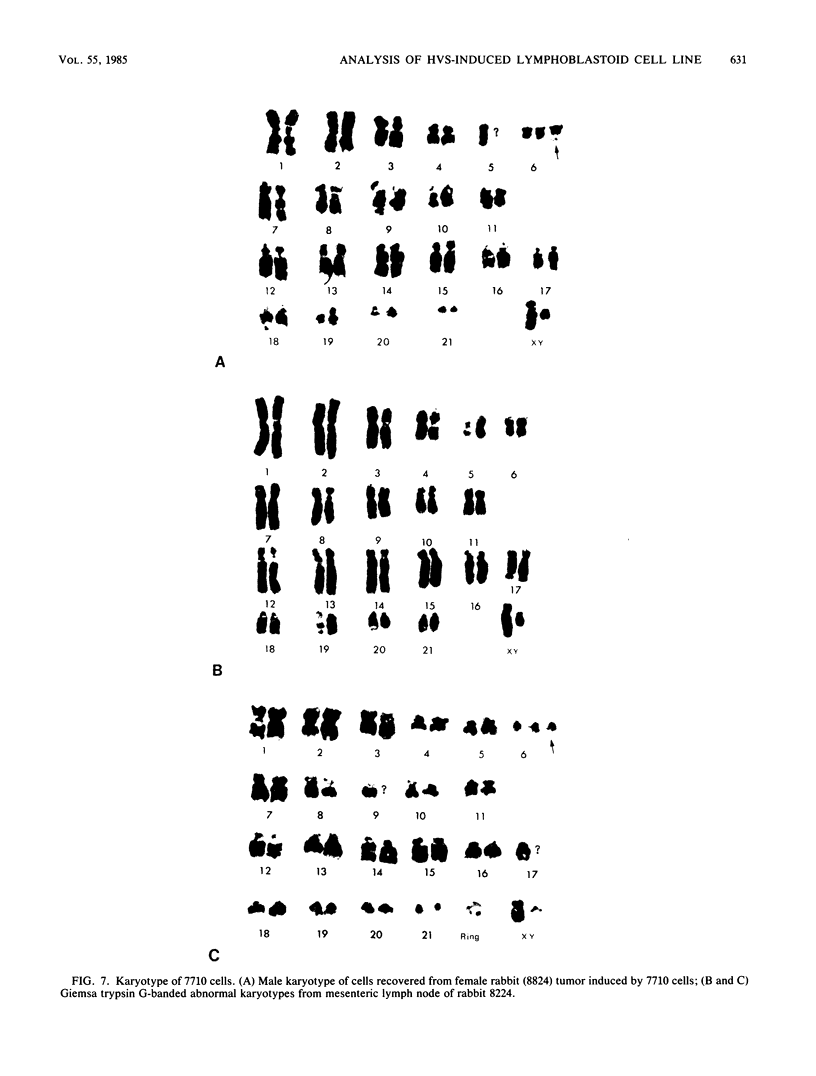
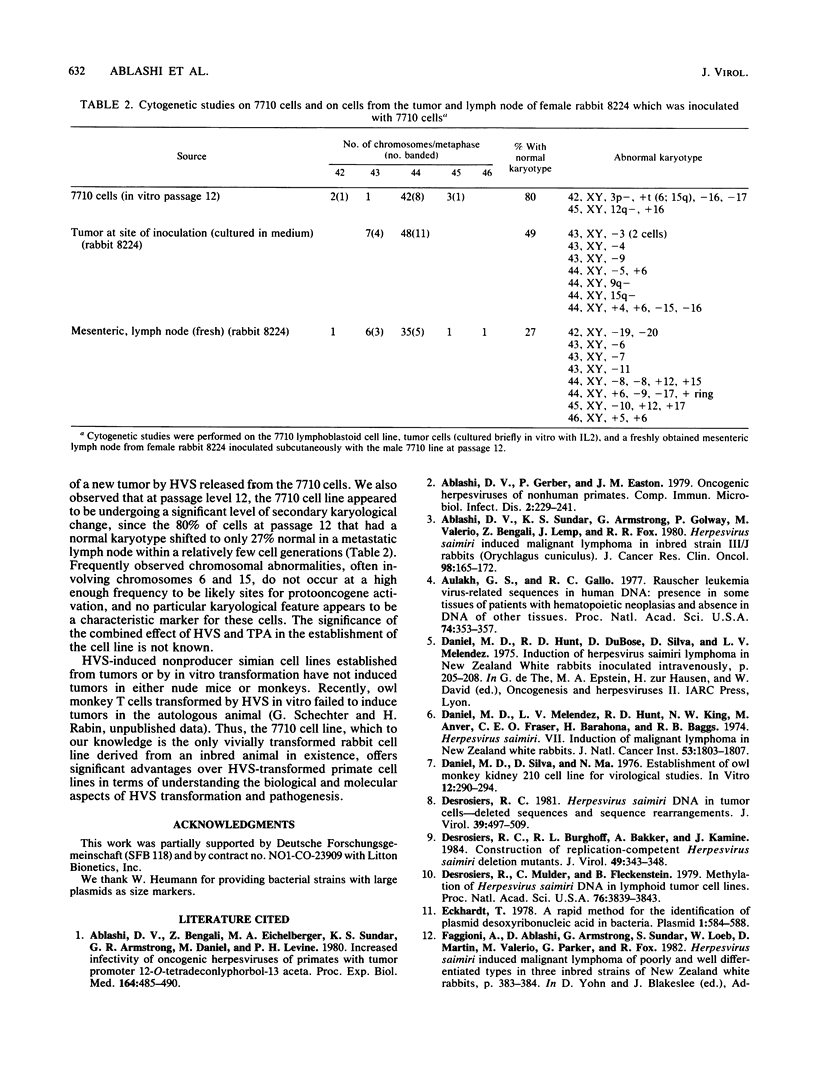

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablashi D. V., Bengali Z. H., Eichelberger M. A., Sundar K. S., Armstrong G. R., Daniel M., Levine P. H. Increased infectivity of oncogenic herpes viruses of primates with tumor promoter 12-O-tetradecanoylphorbol-13-acetate. Proc Soc Exp Biol Med. 1980 Sep;164(4):485–490. doi: 10.3181/00379727-164-40901. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Gerber P., Easton J. Oncogenic herpesviruses of nonhuman primates. Comp Immunol Microbiol Infect Dis. 1979;2(2-3):229–241. doi: 10.1016/0147-9571(79)90011-0. [DOI] [PubMed] [Google Scholar]
- Ablashi D. V., Sundar K. S., Armstrong G., Golway P., Valerio M., Bengali Z., Lemp J., Fox R. R. Herpesvirus saimiri-induced malignant lymphoma in inbred strain III/J rabbits (Oryctolagus cuniculus). J Cancer Res Clin Oncol. 1980;98(2):165–172. doi: 10.1007/BF00405960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulakh G. S., Gallo R. C. Rauscher-leukemia-virus-related sequences in human DNA: presence in some tissues of some patients with hemotopoietic neoplasias and absence in DNA from other tissues. Proc Natl Acad Sci U S A. 1977 Jan;74(1):353–357. doi: 10.1073/pnas.74.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva M. P. Cuidados de enfermagem nos pacientes com "shunt" e fistula artério-venosa. Rev Enferm Nov Dimens. 1976 Nov;2(5):290–294. [PubMed] [Google Scholar]
- Daniel M. D., Hunt R. D., Dubose D., Silva D., Melendez L. V. Induction of herpesvirus saimiri lymphoma in New Zealand white rabbits inoculated intravenously. IARC Sci Publ. 1975;(11 Pt 2):205–208. [PubMed] [Google Scholar]
- Daniel M. D., Meléndez L. V., Hunt R. D., King N. W., Anver M., Fraser C. E., Barahona H., Baggs R. B. Herpesvirus saimiri: VII. Induction of malignant lymphoma in New Zealand white rabbits. J Natl Cancer Inst. 1974 Dec;53(6):1803–1807. [PubMed] [Google Scholar]
- Desrosiers R. C., Burghoff R. L., Bakker A., Kamine J. Construction of replication-competent Herpesvirus saimiri deletion mutants. J Virol. 1984 Feb;49(2):343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. Herpesvirus saimiri DNA in tumor cells--deleted sequences and sequence rearrangements. J Virol. 1981 Aug;39(2):497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C., Mulder C., Fleckenstein B. Methylation of Herpesvirus saimiri DNA in lymphoid tumor cell lines. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3839–3843. doi: 10.1073/pnas.76.8.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt T. A rapid method for the identification of plasmid desoxyribonucleic acid in bacteria. Plasmid. 1978 Sep;1(4):584–588. doi: 10.1016/0147-619x(78)90016-1. [DOI] [PubMed] [Google Scholar]
- Gardella T., Medveczky P., Sairenji T., Mulder C. Detection of circular and linear herpesvirus DNA molecules in mammalian cells by gel electrophoresis. J Virol. 1984 Apr;50(1):248–254. doi: 10.1128/jvi.50.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. R., Jondal M. Herpesvirus ateles and herpesvirus saimiri transform marmoset T cells into continuously proliferating cell lines that can mediate natural killer cell-like cytotoxicity. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6391–6395. doi: 10.1073/pnas.78.10.6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Werner F. J., Bauer I., Fleckenstein B. Structure of nonintegrated, circular Herpesvirus saimiri and Herpesvirus ateles genomes in tumor cell lines and in vitro-transformed cells. J Virol. 1982 Oct;44(1):295–310. doi: 10.1128/jvi.44.1.295-310.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G., Fleckenstein B., Bodemer W. Structural proteins of Herpesvirus saimiri. J Virol. 1983 Sep;47(3):463–470. doi: 10.1128/jvi.47.3.463-470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Pearson G., Rabson A., Ablashi D. V., Falk L., Wolfe L., Dienhardt F., Rabin H. Antibody reactions to herpesvirus saimiri (HVS)-induced early and late antigens (EA and LA) in HVS-infected squirrel, marmoset and owl monkeys. Int J Cancer. 1973 Jul 15;12(1):270–289. doi: 10.1002/ijc.2910120128. [DOI] [PubMed] [Google Scholar]
- Knust E., Dietrich W., Fleckenstein B., Bodemer W. Virus-specific transcription in a Herpesvirus saimiri-transformed lymphoid tumor cell line. J Virol. 1983 Nov;48(2):377–383. doi: 10.1128/jvi.48.2.377-383.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E., Schirm S., Dietrich W., Bodemer W., Kolb E., Fleckenstein B. Cloning of Herpesvirus saimiri DNA fragments representing the entire L-region of the genome. Gene. 1983 Nov;25(2-3):281–289. doi: 10.1016/0378-1119(83)90232-9. [DOI] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marczynska B., Falk L., Wolfe L., Deinhardt F. Transplantation and cytogenetic studies of Herpesvirus saimiri-induced disease in Marmoset monkeys. J Natl Cancer Inst. 1973 Feb;50(2):331–337. doi: 10.1093/jnci/50.2.331. [DOI] [PubMed] [Google Scholar]
- Neubauer R. H., Dunn F. E., Rabin H. Infection of multiple T-cell subsets and changes in lymphocyte functions associated with Herpesvirus saimiri infection of owl monkeys. Infect Immun. 1981 May;32(2):698–706. doi: 10.1128/iai.32.2.698-706.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Desrosiers R. C., Ortaldo J. R., Djeu J. Y., Neubauer R. H. Transformation of owl monkey T cells in vitro with Herpesvirus saimiri. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4563–4567. doi: 10.1073/pnas.81.14.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin H., Hopkins R. F., 3rd, Ruscetti F. W., Neubauer R. H., Brown R. L., Kawakami T. G. Spontaneous release of a factor with properties of T cell growth factor from a continuous line of primate tumor T cells. J Immunol. 1981 Nov;127(5):1852–1856. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Schirm S., Müller I., Desrosiers R. C., Fleckenstein B. Herpesvirus saimiri DNA in a lymphoid cell line established by in vitro transformation. J Virol. 1984 Mar;49(3):938–946. doi: 10.1128/jvi.49.3.938-946.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen W. C., Neubauer R. H., Rabin H. Evidence for suppressor cell activity associated with induction of Herpesvirus saimiri-induced lymphoma. Clin Exp Immunol. 1975 Dec;22(3):468–472. [PMC free article] [PubMed] [Google Scholar]
- Werner F. J., Bornkamm G. W., Fleckenstein B. Episomal viral DNA in a Herpesvirus saimiri-transformed lymphoid cell line. J Virol. 1977 Jun;22(3):794–803. doi: 10.1128/jvi.22.3.794-803.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang-Peng J., Bunn P. A., Jr, Knutsen T., Matthews M. J., Schechter G., Minna J. D. Clinical implications of cytogenetic studies in cutaneous T-cell lymphoma (CTCL). Cancer. 1982 Oct 15;50(8):1539–1553. doi: 10.1002/1097-0142(19821015)50:8<1539::aid-cncr2820500814>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]