Abstract
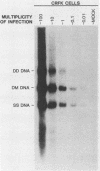
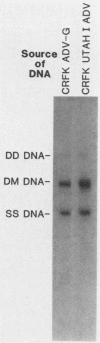
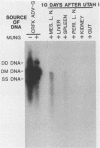
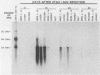
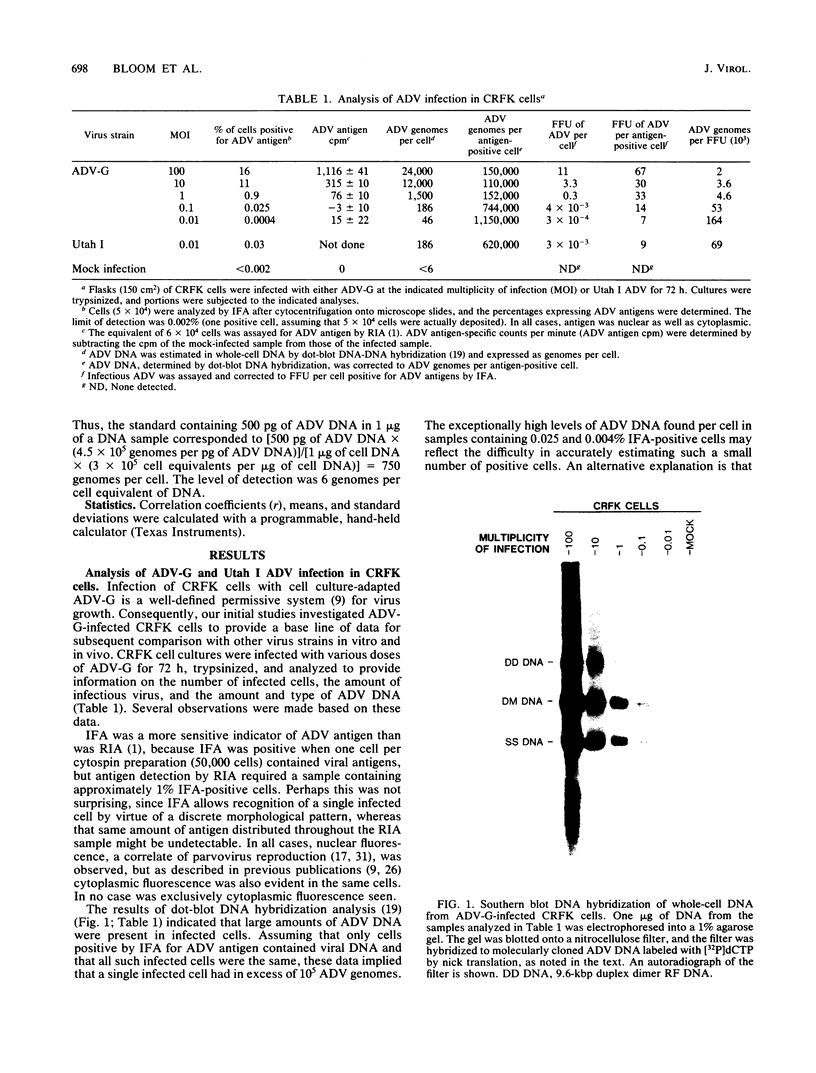
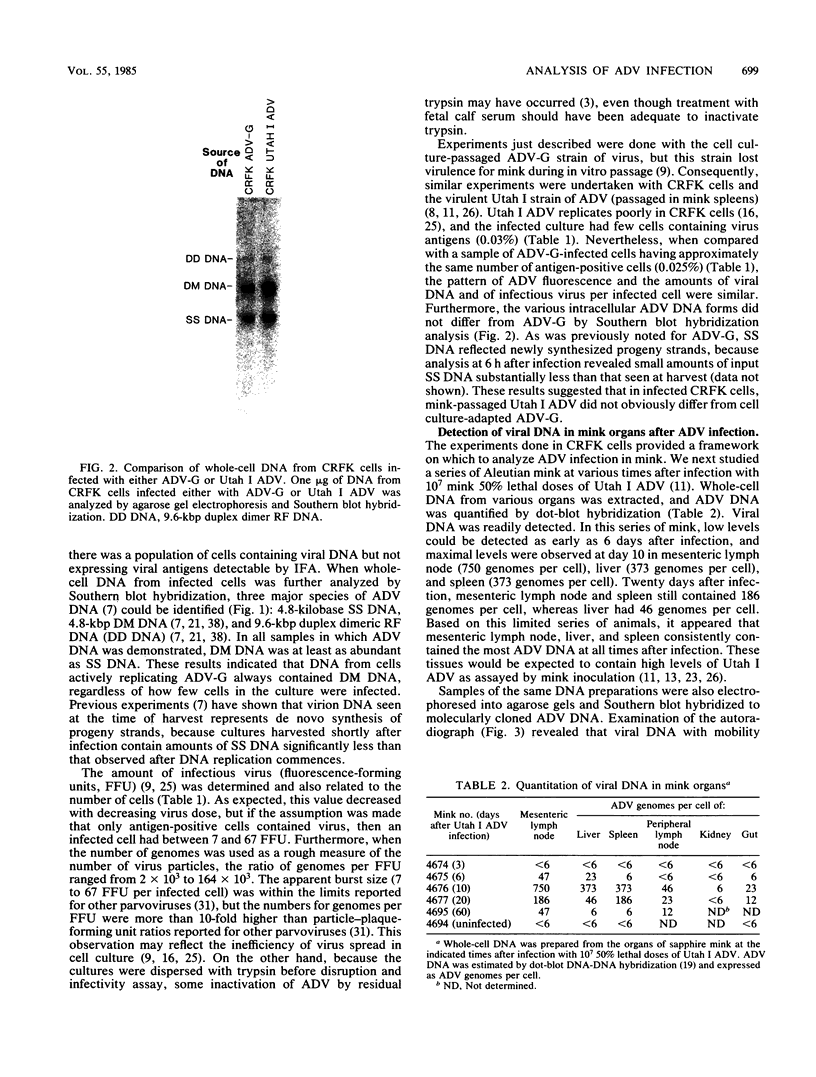
Aleutian disease virus (ADV) infection was analyzed in vivo and in vitro to compare virus replication in cell culture and in mink. Initial experiments compared cultures of Crandell feline kidney (CRFK) cells infected with the avirulent ADV-G strain or the highly virulent Utah I ADV. The number of ADV-infected cells was estimated by calculating the percentage of cells displaying ADV antigen by immunofluorescence (IFA), and several parameters of infection were determined. Infected cells contained large quantities of viral DNA (more than 10(5) genomes per infected cell) as estimated by dot-blot DNA-DNA hybridization, and much of the viral DNA, when analyzed by Southern blot hybridization, was found to be of a 4.8-kilobase-pair duplex monomeric replicative form (DM DNA). Furthermore, the cultures contained 7 to 67 fluorescence-forming units (FFU) per infected cell, and the ADV genome per FFU ratio ranged between 2 X 10(3) and 164 X 10(3). Finally, the pattern of viral antigen detected by IFA was characteristically nuclear, although cytoplasmic fluorescence was often found in the same cells. Because no difference was noted between the two virus strains when cultures containing similar numbers of infected cells were compared, it seemed that both viruses behaved similarly in infected cell culture. These data were used as a basis for the analysis of infection of mink by virulent Utah I ADV. Ten days after infection, the highest levels of viral DNA were detected in spleen (373 genomes per cell), mesenteric lymph node (MLN; 750 genomes per cell), and liver (373 genomes per cell). In marked contrast to infected CRFK cells, the predominant species of ADV DNA in all tissues was single-stranded virion DNA; however, 4.8-kilobase-pair DM DNA was found in MLN and spleen. This observation suggested that MLN and spleen were sites of virus replication, but that the DNA found in liver reflected sequestration of virus produced elsewhere. A final set of experiments examined MLN taken from nine mink 10 days after Utah I ADV infection. All of the nodes contained ADV DNA (46 to 750 genomes per cell), and although single-stranded virion DNA was always the most abundant species, DM DNA was observed. All of the lymph nodes contained virus infectious for CRFK cells, but when the genome per FFU ratio was calculated, virus from the lymph nodes required almost 1,000 times more genomes to produce an FFU than did virus prepared from infected cell cultures.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Bloom M. E. Mink with Aleutian disease have high-affinity antiviral antibodies. Scand J Immunol. 1984 May;19(5):411–418. doi: 10.1111/j.1365-3083.1984.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Aasted B., Bloom M. E. Sensitive radioimmune assay for measuring Aleutian disease virus antigen and antibody. J Clin Microbiol. 1983 Sep;18(3):637–644. doi: 10.1128/jcm.18.3.637-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasted B., Race R. E., Bloom M. E. Aleutian disease virus, a parvovirus, is proteolytically degraded during in vivo infection in mink. J Virol. 1984 Jul;51(1):7–13. doi: 10.1128/jvi.51.1.7-13.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasted B., Tierney G. S., Bloom M. E. Analysis of the quantity of antiviral antibodies from mink infected with different Aleutian disease virus strains. Scand J Immunol. 1984 May;19(5):395–402. doi: 10.1111/j.1365-3083.1984.tb00947.x. [DOI] [PubMed] [Google Scholar]
- Bacterial contamination of human tumor samples. Science. 1984 Aug 17;225(4663):670–671. [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E. Parvovirus infections: features reminiscent of AIDS. Ann N Y Acad Sci. 1984;437:110–120. doi: 10.1111/j.1749-6632.1984.tb37128.x. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Hadlow W. J., Chesebro B. Aleutian disease of mink: the antibody response of sapphire and pastel mink to Aleutian disease virus. J Immunol. 1975 Oct;115(4):1034–1037. [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Kenyon A. J. Anti-deoxyribonucleic acid antibody associated with persistent infection of mink with Aleutian disease virus. Infect Immun. 1980 Aug;29(2):452–458. doi: 10.1128/iai.29.2.452-458.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn E. C., Ramos L., Kenyon A. J. Expression of Aleutian mink disease antigen in cell culture. Infect Immun. 1977 Jan;15(1):204–211. doi: 10.1128/iai.15.1.204-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S., Alexandersen S., Lund E., Have P., Hansen M. Acute interstitial pneumonitis caused by Aleutian disease virus in mink kits. Acta Pathol Microbiol Immunol Scand A. 1984 Sep;92(5):391–393. doi: 10.1111/j.1699-0463.1984.tb04419.x. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Aasted B., Garon C. F., Bloom M. E. Molecular cloning of the Aleutian disease virus genome: expression of Aleutian disease virus antigens by a recombinant plasmid. J Virol. 1983 Dec;48(3):573–579. doi: 10.1128/jvi.48.3.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Porter H. G., Larsen A. E. Much of the increased IgG in Aleutian disease of mink is viral antibody. J Exp Pathol. 1984;1(2):79–88. [PubMed] [Google Scholar]
- Race R. E., Bloom M. E., Coe J. E. Demonstration of Aleutian disease virus-specific lymphocyte response in mink with progressive Aleutian disease: comparison of sapphire and pastel mink infected with different virus strains. J Immunol. 1983 Sep;131(3):1558–1564. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ron D., Tattersall P., Tal J. Formation of a host range mutant of the lymphotropic strain of minute virus of mice during persistent infection in mouse L cells. J Virol. 1984 Oct;52(1):63–69. doi: 10.1128/jvi.52.1.63-69.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S., Kaaden O. R., van Dawen S., Moennig V. Aleutian disease virus in B and T lymphocytes from blood and spleen and in bone marrow cells from naturally infected mink. Intervirology. 1984;22(4):211–217. doi: 10.1159/000149553. [DOI] [PubMed] [Google Scholar]
- Shahrabadi M. S., Cho H. J. Detection and localization of aleutian disease virus and its antigens in vivo by immunoferritin technique. Can J Comp Med. 1977 Oct;41(4):435–445. [PMC free article] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Virgin H. W., 4th, Unanue E. R. Suppression of the immune response to Listeria monocytogenes. I. Immune complexes inhibit resistance. J Immunol. 1984 Jul;133(1):104–109. [PubMed] [Google Scholar]