Abstract
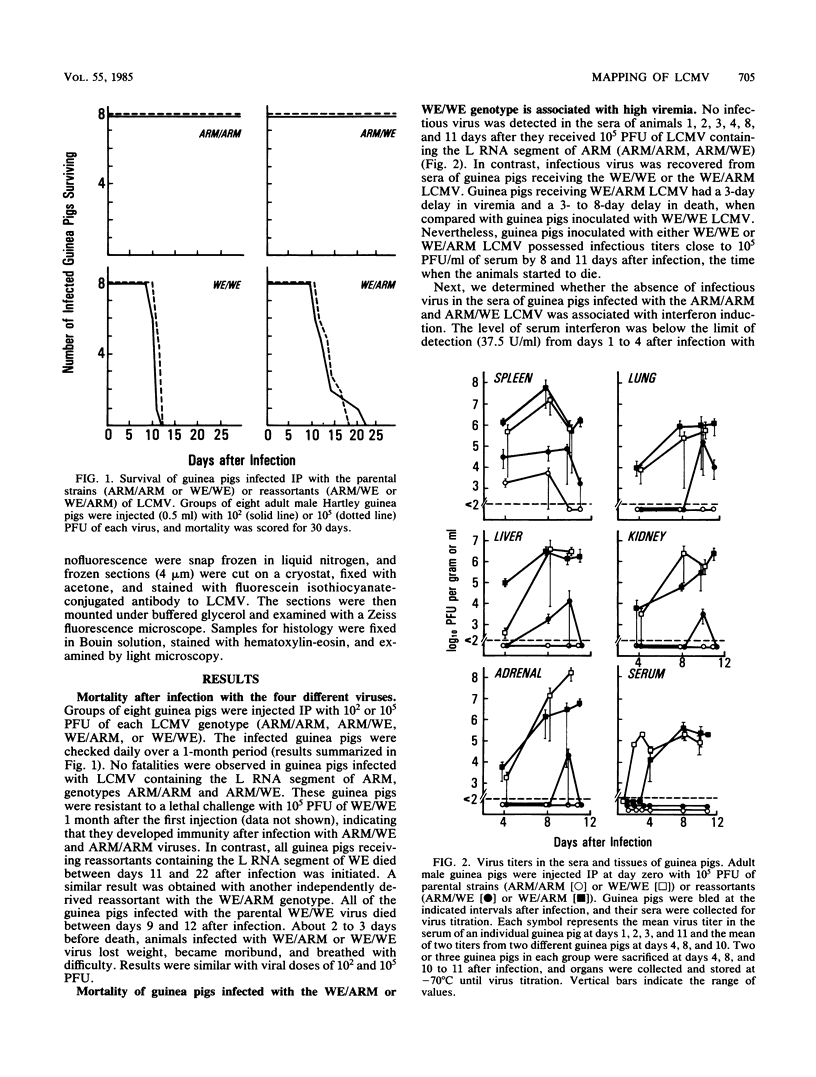
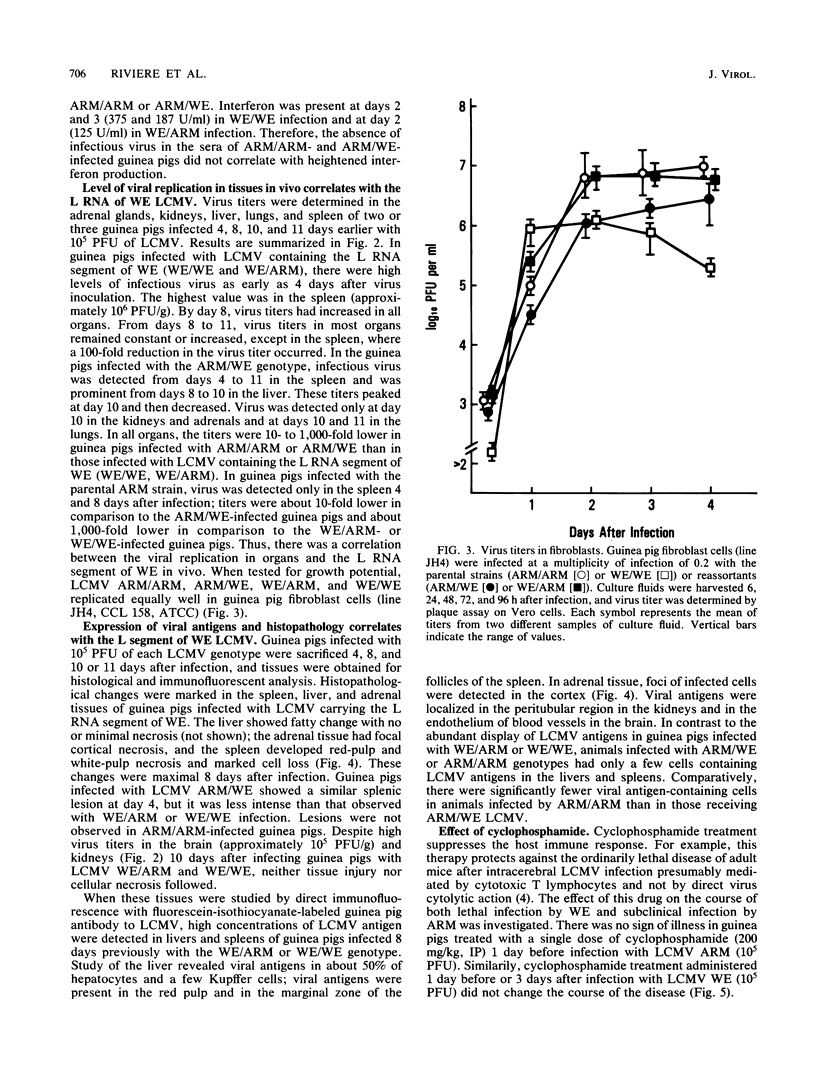
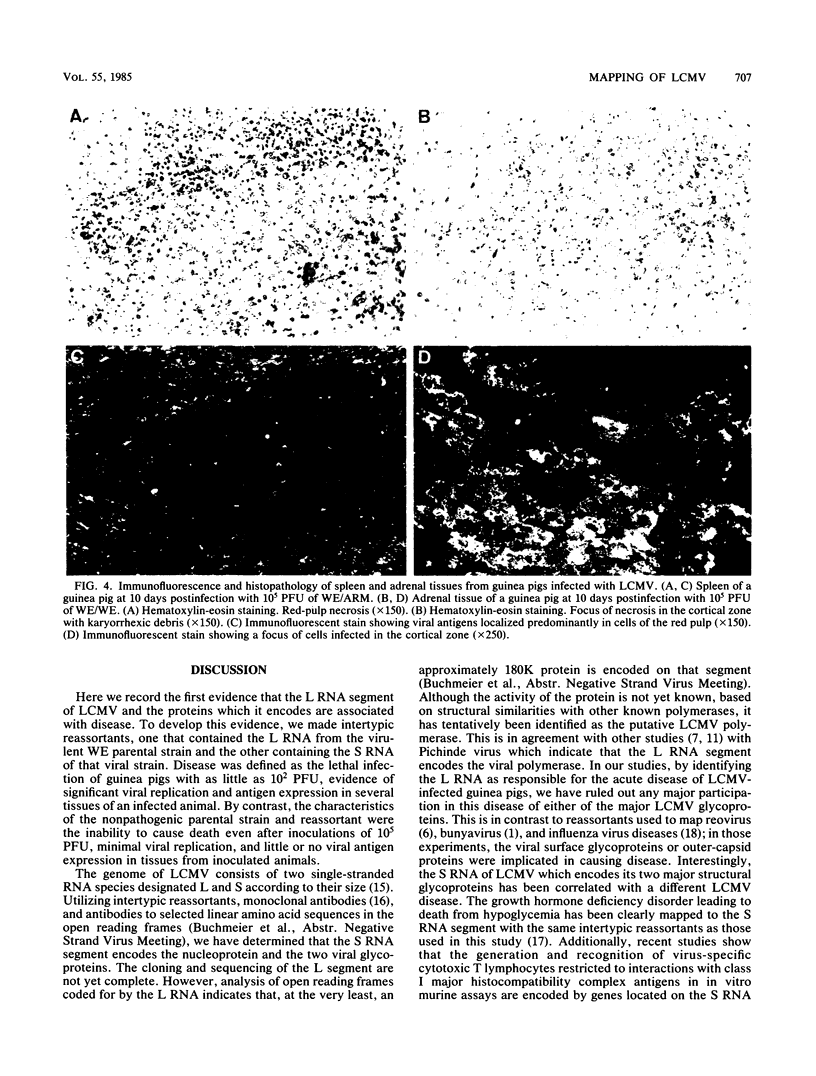
The Armstrong CA 1371 (ARM) and WE strains of lymphocytic choriomeningitis virus (LCMV) differ in the ability to produce disease in adult guinea pigs. Infection with the ARM strain is not lethal, even at high virus doses (greater than 10,000 PFU), whereas the WE strain causes 100% mortality even at low doses (less than 10 PFU). To determine the genetic basis of this virulence, intertypic reassortants were made between the ARM and WE strains of LCMV. The two reassortants with the genotypes WE/ARM (L segment of WE and S segment of ARM) and ARM/WE (L segment of ARM and S segment of WE) were tested for their pathogenicity in guinea pigs. The ARM/WE reassortant was avirulent like the ARM/ARM parental strain. Minimal viral replication was observed in organs of guinea pigs inoculated with 10(2) or 10(5) PFU of ARM/ARM or ARM/WE, and all animals survived. In contrast, the WE/ARM reassortant was highly virulent like the WE/WE parental strain and killed all of the infected animals. High levels of viral replication were observed in guinea pigs infected with the latter two strains. In contrast to these in vivo observations, both the parental strains and the ARM/WE or WE/ARM reassortants had similar growth potential in cultured guinea pig fibroblasts. Thus, the L RNA segment of LCMV WE is important for viral replication in vivo and is associated with fatal acute disease after infection of adult guinea pigs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchmeier M. J., Lewicki H. A., Tomori O., Oldstone M. B. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology. 1981 Aug;113(1):73–85. doi: 10.1016/0042-6822(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Fields B. N., Greene M. I. Genetic and molecular mechanisms of viral pathogenesis: implications for prevention and treatment. Nature. 1982 Nov 4;300(5887):19–23. doi: 10.1038/300019a0. [DOI] [PubMed] [Google Scholar]
- Harnish D. G., Dimock K., Bishop D. H., Rawls W. E. Gene mapping in Pichinde virus: assignment of viral polypeptides to genomic L and S RNAs. J Virol. 1983 May;46(2):638–641. doi: 10.1128/jvi.46.2.638-641.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk W. E., Cash P., Peters C. J., Bishop D. H. Formation and characterization of an intertypic lymphocytic choriomeningitis recombinant virus. J Gen Virol. 1980 Nov;51(Pt 1):213–218. doi: 10.1099/0022-1317-51-1-213. [DOI] [PubMed] [Google Scholar]
- Leung W. C., Leung M. F., Rawls W. E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979 Apr;30(1):98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C. A., Tosolini F. A. Pathogenesis of lesions in lymphoid tissue of mice infected with lymphocytic choriomeningitis (LCM) virus. Br J Exp Pathol. 1969 Dec;50(6):584–592. [PMC free article] [PubMed] [Google Scholar]
- Mogensen S. C. Role of macrophages in natural resistance to virus infections. Microbiol Rev. 1979 Mar;43(1):1–26. doi: 10.1128/mr.43.1.1-26.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F. A., Buchmeier M. J., Rawls W. E. The reticuloendothelium as the target in a virus infection. Pichinde virus pathogenesis in two strains of hamsters. Lab Invest. 1977 Nov;37(5):502–515. [PubMed] [Google Scholar]
- Pedersen I. R. Structural components and replication of arenaviruses. Adv Virus Res. 1979;24:277–330. doi: 10.1016/s0065-3527(08)60396-6. [DOI] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Dutko F. J., Oldstone M. B. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985 Mar;53(3):966–968. doi: 10.1128/jvi.53.3.966-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P., Oldstone M. B. Perturbation of differentiated functions during viral infection in vivo. II. Viral reassortants map growth hormone defect to the S RNA of the lymphocytic choriomeningitis virus genome. Virology. 1985 Apr 15;142(1):175–182. doi: 10.1016/0042-6822(85)90431-3. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Influenza virus genetics. Adv Genet. 1979;20:1–36. doi: 10.1016/s0065-2660(08)60544-1. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]




