Abstract
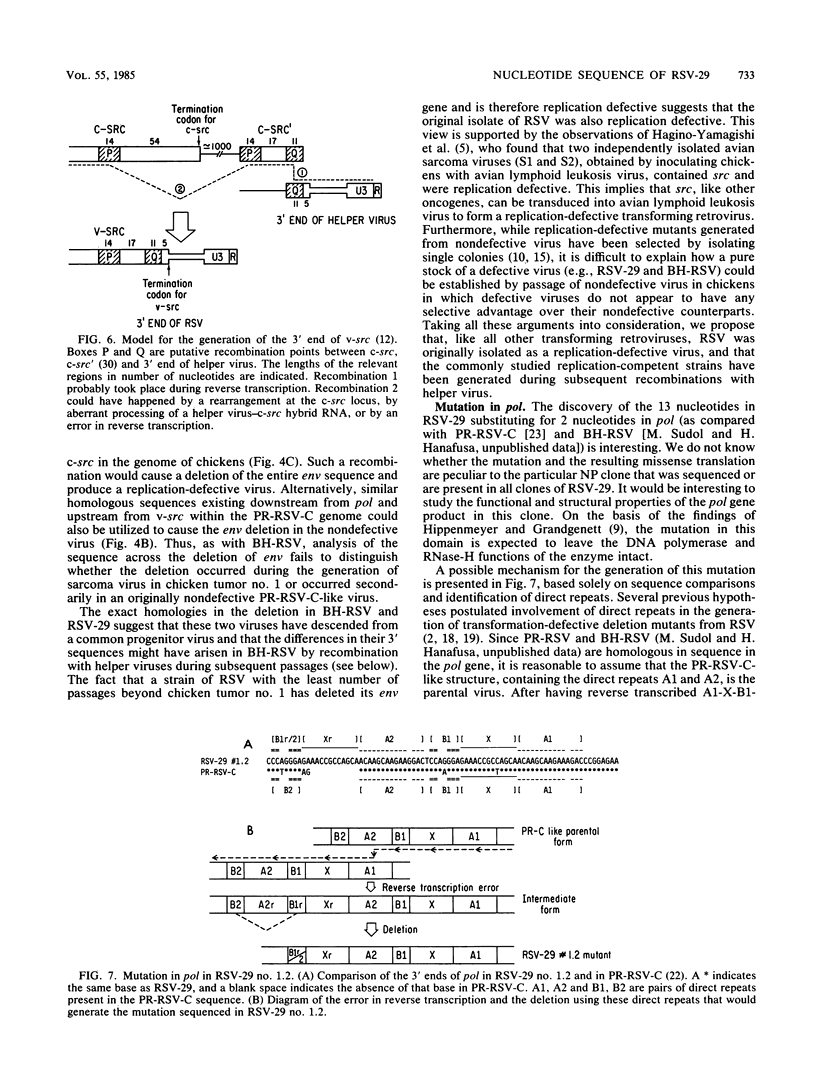
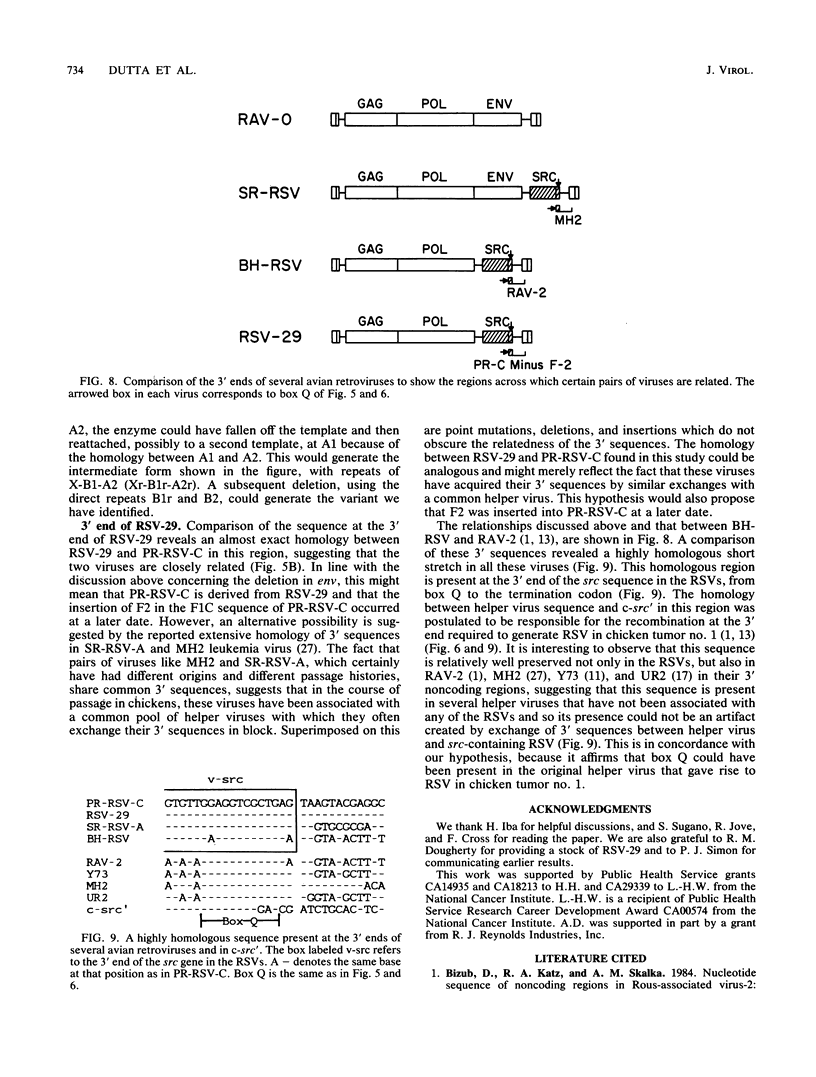
Rous sarcoma virus-29 (RSV-29) is the strain of RSV that has the least number of passages beyond its isolation from chicken tumor no. 1 among all current strains of RSV. Biological characterization indicated that it was replication defective. RNA analysis of nonproducer clones of RSV-29-infected chicken embryonic fibroblasts showed the presence of a subgenomic message of 2.6 kilobases containing src and a genomic RNA of 7.7 kilobases that contains gag, pol, and src, but not env. The src-containing EcoRI fragment of RSV-29 proviral DNA was molecularly cloned. Sequence analysis of the regions flanking src revealed that the env gene was completely deleted in RSV-29 and that the sequence across the deletion was exactly the same as the Bryan high-titer strain of RSV. The sequence immediately 3' to src in RSV-29 was closely related to that of the Prague strain of RSV. The fact that the strain of RSV which has the minimal number of passages beyond its isolation is replication defective supports the hypothesis of Lerner and Hanafusa (J. Virol. 49:549-556, 1984) that the original RSV is a defective transforming virus. This defective transforming virus is postulated to be the precursor to other defective RSVs like the Bryan high-titer strain and to nondefective RSVs like the Prague strain. The particular clone of RSV-29 that we studied also had a short stretch of sequence duplication at the 3' end of the pol gene, which was presumably created by an error of reverse transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bizub D., Katz R. A., Skalka A. M. Nucleotide sequence of noncoding regions in Rous-associated virus-2: comparisons delineate conserved regions important in replication and oncogenesis. J Virol. 1984 Feb;49(2):557–565. doi: 10.1128/jvi.49.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Kawai S., Wang L. H., Vogt P. K., Murphy H. M., Hanafusa H. RNA of replication-defective strains of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1569–1573. doi: 10.1073/pnas.72.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H. ANALYSIS OF THE DEFECTIVENESS OF ROUS SARCOMA VIRUS. 3. DETERMINING INFLUENCE OF A NEW HELPER VIRUS ON THE HOST RANGE AND SUSCEPTIBILITY TO INTERFERENCE OF RSV. Virology. 1965 Feb;25:248–255. doi: 10.1016/0042-6822(65)90203-5. [DOI] [PubMed] [Google Scholar]
- HANAFUSA H., HANAFUSA T., RUBIN H. The defectiveness of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1963 Apr;49:572–580. doi: 10.1073/pnas.49.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagino-Yamagishi K., Ikawa S., Kawai S., Hihara H., Yamamoto T., Toyoshima K. Characterization of two strains of avian sarcoma virus isolated from avian lymphatic leukosis virus-induced sarcomas. Virology. 1984 Sep;137(2):266–275. doi: 10.1016/0042-6822(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Hanafusa H. Rapid transformation of cells by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1969 Jun;63(2):318–325. doi: 10.1073/pnas.63.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippenmeyer P. J., Grandgenett D. P. Requirement of the avian retrovirus pp32 DNA binding protein domain for replication. Virology. 1984 Sep;137(2):358–370. doi: 10.1016/0042-6822(84)90228-9. [DOI] [PubMed] [Google Scholar]
- KLEMENT V., SVOBODA J. Induction of tumours in Syrian hamsters by two variants of Rous sarcoma virus. Folia Biol (Praha) 1963 Jun;9:181–188. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. Isolation of defective mutant of avian sarcoma virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3493–3497. doi: 10.1073/pnas.70.12.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Lerner T. L., Hanafusa H. DNA sequence of the Bryan high-titer strain of Rous sarcoma virus: extent of env deletion and possible genealogical relationship with other viral strains. J Virol. 1984 Feb;49(2):549–556. doi: 10.1128/jvi.49.2.549-556.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. S., Radke K., Hughes S., Quintrell N., Bishop J. M., Varmus H. E. Mutants of Rous sarcoma virus with extensive deletions of the viral genome. Virology. 1979 Jul 30;96(2):530–546. doi: 10.1016/0042-6822(79)90109-0. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Neckameyer W. S., Wang L. H. Nucleotide sequence of avian sarcoma virus UR2 and comparison of its transforming gene with other members of the tyrosine protein kinase oncogene family. J Virol. 1985 Mar;53(3):879–884. doi: 10.1128/jvi.53.3.879-884.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer C. A., Pogue-Geile K., Guntaka R., Staskus K. A., Faras A. J. Involvement of directly repeated sequences in the generation of deletions of the avian sarcoma virus src gene. J Virol. 1983 Aug;47(2):380–382. doi: 10.1128/jvi.47.2.380-382.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin J. D., Wang L. H. Mechanisms for the generation of src-deletion mutants and recovered sarcoma viruses: identification of viral sequences involved in src deletions and in recombination with c-src sequences. Virology. 1984 Oct 30;138(2):236–245. doi: 10.1016/0042-6822(84)90348-9. [DOI] [PubMed] [Google Scholar]
- SCHMIDT-RUPPIN K. H. HETEROTRANSPLANTATION OF ROUS SARCOMA AND ROUS SARCOMA VIRUS TO MAMMALS. Oncology. 1964;17:247–272. doi: 10.1159/000224235. [DOI] [PubMed] [Google Scholar]
- SIMONS P. J., DOUGHERTY R. M. ANTIGENIC CHARACTERISTICS OF THREE VARIANTS OF ROUS SARCOMA VIRUS. J Natl Cancer Inst. 1963 Nov;31:1275–1283. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H. Nucleotide sequence of Fujinami sarcoma virus: evolutionary relationship of its transforming gene with transforming genes of other sarcoma viruses. Cell. 1982 Oct;30(3):787–795. doi: 10.1016/0092-8674(82)90283-5. [DOI] [PubMed] [Google Scholar]
- Sutrave P., Jansen H. W., Bister K., Rapp U. R. 3'-Terminal region of avian carcinoma virus MH2 shares sequence elements with avian sarcoma viruses Y73 and SR-A. J Virol. 1984 Nov;52(2):703–705. doi: 10.1128/jvi.52.2.703-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. II. Comparison of the src genes of two strains of avian sarcoma virus and of the cellular homolog. J Virol. 1982 Oct;44(1):12–18. doi: 10.1128/jvi.44.1.12-18.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H., Junghans R. P., Ju G., Skalka A. M. Comparison between the viral transforming gene (src) of recovered avian sarcoma virus and its cellular homolog. Mol Cell Biol. 1981 Nov;1(11):1024–1037. doi: 10.1128/mcb.1.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Wang L. H., Duesberg P., Beemon K., Vogt P. K. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975 Oct;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Feldman R., Shibuya M., Hanafusa H., Notter M. F., Balduzzi P. C. Genetic structure, transforming sequence, and gene product of avian sarcoma virus UR1. J Virol. 1981 Oct;40(1):258–267. doi: 10.1128/jvi.40.1.258-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]