Abstract
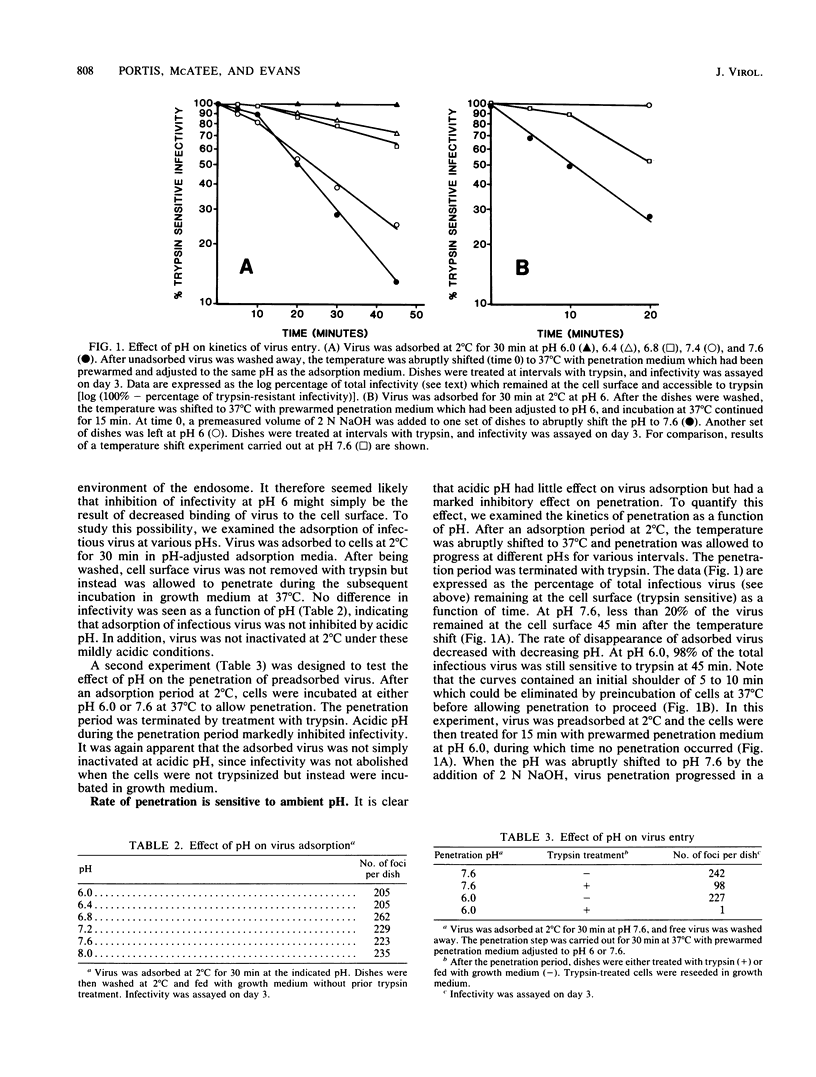
The entry into cells by many enveloped RNA viruses is accomplished by endocytosis and subsequent penetration of the endosomal membrane by an acidic pH-dependent fusion event. In the current study, we examined early events in the infectious entry of mouse retroviruses, using as a framework the observation that infection of a mouse tail skin cell line by the ecotropic virus Friend murine leukemia virus was inhibited at mildly acidic pH (pH 6). This inhibition operated on a postadsorption step, since binding of virus was unaffected at this pH. The rate of penetration of preadsorbed virus, which displayed first-order kinetics, was markedly affected by changes in the pH of the medium. The half-time for disappearance of infectious cell surface virus at 37 degrees C was approximately 10 min at pH 7.6. At pH 6.0, however, greater than 98% of the adsorbed infectivity remained at the cell surface after 45 min. This cell surface virus, though not infecting the cell at pH 6.0, retained its capacity to enter and infect the cell when the pH of the medium was raised. Acidic pH had little effect on the rate of fluid uptake by the cells, as measured by internalization of [3H]sucrose, indicating that global inhibition of endocytosis had not occurred. In contrast, cell fusion induced by Friend murine leukemia virus was optimal at pH 7.6 but markedly inhibited at a pH of less than 6.4. This inhibitory effect of acidic pH on membrane fusion is unique among the enveloped viruses which have been studied and would preclude entry of Friend murine leukemia virus from within acidified endocytic vesicles. Entry of other members of the ecotropic, mink cell focus-forming, and xenotropic host range groups displayed similar pH sensitivity. However, one xenotropic virus was relatively resistant to the effect of acidic pH, suggesting that differences might exist in the requirements for entry of different retroviruses.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., Nexø B. A. Entry of murine retrovirus into mouse fibroblasts. Virology. 1983 Feb;125(1):85–98. doi: 10.1016/0042-6822(83)90065-x. [DOI] [PubMed] [Google Scholar]
- Bishayee S., Strand M., August J. T. Cellular membrane receptors for oncovirus envelope glycoprotein: properties of the binding reaction and influence of different reagents on the substrate and the receptors. Arch Biochem Biophys. 1978 Jul;189(1):161–171. doi: 10.1016/0003-9861(78)90129-7. [DOI] [PubMed] [Google Scholar]
- Bister K., Varmus H. E., Stavnezer E., Hunter E., Vogt P. K. Biological and biochemical studies on the inactivation of avian oncoviruses by ultraviolet irradiation. Virology. 1977 Apr;77(2):689–704. doi: 10.1016/0042-6822(77)90492-5. [DOI] [PubMed] [Google Scholar]
- Bratt M. A., Gallaher W. R. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc Natl Acad Sci U S A. 1969 Oct;64(2):536–543. doi: 10.1073/pnas.64.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Britt W., Evans L., Wehrly K., Nishio J., Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983 May;127(1):134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Cloyd M., Britt W., Portis J., Collins J., Nishio J. Characterization of mouse monoclonal antibodies specific for Friend murine leukemia virus-induced erythroleukemia cells: friend-specific and FMR-specific antigens. Virology. 1981 Jul 15;112(1):131–144. doi: 10.1016/0042-6822(81)90619-x. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K. Different murine cell lines manifest unique patterns of interference to superinfection by murine leukemia viruses. Virology. 1985 Feb;141(1):119–129. doi: 10.1016/0042-6822(85)90188-6. [DOI] [PubMed] [Google Scholar]
- Clavell L. A., Bratt M. A. Hemolytic interaction of Newcastle disease virus and chicken erythrocytes. II. Determining factors. Appl Microbiol. 1972 Mar;23(3):461–470. doi: 10.1128/am.23.3.461-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLarco J., Todaro G. J. Membrane receptors for murine leukemia viruses: characterization using the purified viral envelope glycoprotein, gp71. Cell. 1976 Jul;8(3):365–371. doi: 10.1016/0092-8674(76)90148-3. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Sefton B. M. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell. 1978 Nov;15(3):985–992. doi: 10.1016/0092-8674(78)90282-9. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Miyamoto T., Hanafusa H. A type of chick embryo cell that fails to support formation of infectious RSV. Virology. 1970 Jan;40(1):55–64. doi: 10.1016/0042-6822(70)90378-8. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P., Nicolaiew N., DesGroseillers L., Rassart E. Molecular cloning of infectious viral DNA from ecotropic neurotropic wild mouse retrovirus. J Virol. 1983 Mar;45(3):1159–1163. doi: 10.1128/jvi.45.3.1159-1163.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Koch W., Hunsmann G., Friedrich R. Nucleotide sequence of the envelope gene of Friend murine leukemia virus. J Virol. 1983 Jan;45(1):1–9. doi: 10.1128/jvi.45.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacIntyre E. H., Wintersgill C. J., Thormar H. Morphological transformation of human astrocytes by visna virus with complete virus production. Nat New Biol. 1972 May 24;237(73):111–113. doi: 10.1038/newbio237111a0. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Mathieu-Mahul D., Heard J. M., Fichelson S., Gisselbrecht S., Sola B., Larsen C. J. Viral expression in two myelomonocytic cell lines obtained from long-term bone marrow culture infected with the Friend polycythemia-inducing virus (FV-P). Virology. 1982 May;119(1):59–67. doi: 10.1016/0042-6822(82)90065-4. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Gilden R. V. Electron microscopic studies of tumor viruses. I. Entry of murine leukemia virus into mouse embryo fibroblasts. J Virol. 1971 Mar;7(3):395–406. doi: 10.1128/jvi.7.3.395-406.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Hamaguchi M., Toyoda T., Yoshida T. The uncoating of paramyxoviruses may not require a low pH mediated step. Virology. 1983 Oct 15;130(1):263–268. doi: 10.1016/0042-6822(83)90138-1. [DOI] [PubMed] [Google Scholar]
- Nagy K., Clapham P., Cheingsong-Popov R., Weiss R. A. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int J Cancer. 1983 Sep 15;32(3):321–328. doi: 10.1002/ijc.2910320310. [DOI] [PubMed] [Google Scholar]
- Pinter A., Lieman-Hurwitz J., Fleissner E. The nature of the association between the murine leukemia virus envelope proteins. Virology. 1978 Dec;91(2):345–351. doi: 10.1016/0042-6822(78)90382-3. [DOI] [PubMed] [Google Scholar]
- Piraino F. The mechanism of genetic resistance of chick embryo cells to infection by Rous sarcoma virus-Bryan strain (BS-RSV). Virology. 1967 Aug;32(4):700–707. doi: 10.1016/0042-6822(67)90046-3. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J., Cloyd M. W. Monoclonal antibodies to xenotropic and MCF murine leukemia viruses derived during the graft-versus-host reaction. Virology. 1982 Apr 15;118(1):181–190. doi: 10.1016/0042-6822(82)90331-2. [DOI] [PubMed] [Google Scholar]
- Portis J. L., McAtee F. J. Monoclonal antibodies derived during graft-versus-host reaction. II. Antibodies detect unique determinants common to many MCF viruses. Virology. 1983 Apr 15;126(1):96–105. doi: 10.1016/0042-6822(83)90464-6. [DOI] [PubMed] [Google Scholar]
- Redmond S., Peters G., Dickson C. Mouse mammary tumor virus can mediate cell fusion at reduced pH. Virology. 1984 Mar;133(2):393–402. doi: 10.1016/0042-6822(84)90405-7. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Entry of the toxic proteins abrin, modeccin, ricin, and diphtheria toxin into cells. II. Effect of pH, metabolic inhibitors, and ionophores and evidence for toxin penetration from endocytotic vesicles. J Biol Chem. 1982 Jul 10;257(13):7504–7513. [PubMed] [Google Scholar]
- Schneider J., Falk H., Hunsmann G. Virosomes constructed from lipid and purified Friend leukaemia virus glycoprotein. J Gen Virol. 1983 Mar;64(Pt 3):559–565. doi: 10.1099/0022-1317-64-3-559. [DOI] [PubMed] [Google Scholar]
- Sitbon M., Nishio J., Wehrly K., Lodmell D., Chesebro B. Use of a focal immunofluorescence assay on live cells for quantitation of retroviruses: distinction of host range classes in virus mixtures and biological cloning of dual-tropic murine leukemia viruses. Virology. 1985 Feb;141(1):110–118. doi: 10.1016/0042-6822(85)90187-4. [DOI] [PubMed] [Google Scholar]
- Sung S. S., Nelson R. S., Silverstein S. C. The role of the mannose/N-acetylglucosamine receptor in the pinocytosis of horseradish peroxidase by mouse peritoneal macrophages. J Cell Physiol. 1983 Jul;116(1):21–25. doi: 10.1002/jcp.1041160105. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- White J., Kielian M., Helenius A. Membrane fusion proteins of enveloped animal viruses. Q Rev Biophys. 1983 May;16(2):151–195. doi: 10.1017/s0033583500005072. [DOI] [PubMed] [Google Scholar]
- White J., Matlin K., Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981 Jun;89(3):674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarling D. A., Keshet I. Fusion activity of virions of murine leukemia virus. Virology. 1979 May;95(1):185–196. doi: 10.1016/0042-6822(79)90413-6. [DOI] [PubMed] [Google Scholar]



