Abstract
cDNA clones encoding a novel protein (VAMP5) homologous to synaptobrevins/VAMPs are detected during database searches. The predicted 102–amino acid VAMP5 harbors a 23-residue hydrophobic region near the carboxyl terminus and exhibits an overall amino acid identity of 33% with synaptobrevin/VAMP1 and 2 and cellubrevin. Northern blot analysis reveals that the mRNA for VAMP5 is preferentially expressed in the skeletal muscle and heart, whereas significantly lower levels are detected in several other tissues but not in the brain. During in vitro differentiation (myogenesis) of C2C12 myoblasts into myotubes, the mRNA level for VAMP5 is increased ∼8- to 10-fold. Immunoblot analysis using antibodies specific for VAMP5 shows that the protein levels are also elevated ∼6-fold during in vitro myogenesis of C2C12 cells. Indirect immunofluorescence microscopy and immunoelectron microscopy reveal that VAMP5 is associated with the plasma membrane as well as intracellular perinuclear and peripheral vesicular structures of myotubes. Epitope-tagged versions of VAMP5 are similarly targeted to the plasma membrane.
INTRODUCTION
Vesicle-mediated protein transport is the major mechanism for protein movement along the secretory and endocytotic pathways and for regulated protein secretion and neurotransmitter release (Palade, 1975; Pryer et al., 1992; Rothman, 1994; Hong, 1996; Rothman and Wieland, 1996; Schekman and Orci, 1996). Because of the existence of diverse intracellular organelles and their resulting vesicles, understanding the molecular mechanisms that govern the specific docking and fusion of vesicles with their acceptor compartment represents an important aspect in our current studies of membrane traffic (Rothman and Warren, 1994; Scheller, 1995; Südhof 1995; Pfeffer, 1996). The SNARE hypothesis suggests that vesicle docking and fusion are mediated by specific interaction between vesicle-associated proteins termed v-SNAREs and those (termed t-SNAREs) associated with the target membrane (Söllner et al., 1993; Ferro-Novick and Jahn, 1994; Rothman, 1994; Rothman and Warren, 1994; Scheller, 1995; Südhof, 1995; Whiteheart and Kubalek, 1995; Pfeffer, 1996; Weber et al., 1998). In the neuron, synaptobrevin/VAMP1 functions as the v-SNARE for synaptic vesicles, whereas syntaxin 1 and SNAP-25 associated with the presynaptic membrane function as t-SNAREs (Söllner et al., 1993). The specific interaction of synaptobrevin/VAMP1 with a protein complex composed of syntaxin 1 and SNAP-25 plays a fundamental role in docking and fusion of synaptic vesicles and for regulated neurotransmitter release (Söllner et al., 1993; Scheller, 1995; Südhof, 1995).
The identification of novel proteins homologous to synaptobrevins/VAMPs, syntaxin 1 and SNAP-25, establishes that they each represent members of distinct protein families. Currently, the synaptobrevin family contains three members. Synaptobrevin 1 and 2 are highly homologous (∼80% amino acid identities) proteins associated with synaptic vesicles and secretory granules and are involved in regulated protein secretion and neurotransmitter release (Söllner et al., 1993; Scheller, 1995; Südhof, 1995), whereas cellubrevin is ubiquitously expressed and is associated with the endocytotic pathway (McMahon et al., 1993). Mammalian proteins distantly related to synaptobrevins have also been characterized, and these include rbet1, sec22a, sec22b/ERS-24, sec22c, and hYKT6 (Hay et al., 1996, 1997; McNew et al., 1997; Paek et al., 1997; Zhang et al., 1997; Tang et al., 1998a). Ten distinct syntaxin-like proteins (syntaxin 1, 2, 3, 4, 5, 6, 7, 10, 12, and 16) and three SNAP-25-like proteins (SNAP-25a, SNAP-25b, and SNAP-23) have been characterized (Oyler et al., 1989; Bennett et al., 1992, 1993; Bock et al., 1996, 1997; Ravichandran et al., 1996; Wang et al., 1997; Tang et al., 1998b–d; Wong et al., 1998). The existence of other potential members of the syntaptobrevin/VAMP, syntaxin, and SNAP-25 protein families have been suggested, and their molecular and biochemical characterizations remain to be conducted (Bock and Scheller, 1997; our unpublished observations).
Myogenesis represents one of the few well-studied models of cellular differentiation, and several types of myoblasts have been used for in vitro myogenesis. Genetically, the muscle-specific basic helix–loop–helix (bHLH) transcription factors MyoD, myogenin, Myf5, and MRF4 play a determining role in the commitment to and in the actual process of myogenesis (Lassar et al., 1994; Tam et al., 1995; Molkentin and Olson, 1996; Rawls and Olson, 1997; Walsh and Perlman, 1997). These myogenic basic HLH proteins interact with ubiquitously expressed HLH proteins such as E12 and E47 to form functional heterodimers, which bind to the E box consensus sequence (CANNTG). Another family of transcriptional factors that participate in myogenesis are myocyte enhancer factors (MEF2), which are encoded by a family of four genes whose products share sequence homology within a 56–amino acid MADS domain. MEF2 factors bind DNA either as homodimers or heterodimers between various MEF2 members. Both the E box site and MEF2 binding site have been identified in promoters and/or enhancers of many muscle-specific genes. Another important discovery is that the expression of the cyclin-dependent kinase inhibitor p21/Cip1/WAF1 is greatly enhanced during myogenesis, and this plays a critical role in withdrawal of differentiating myoblasts from the cell cycle (Walsh and Perlman, 1997). During in vitro myogenesis, p21 induction and cell cycle withdrawal are downstream of enhanced expression of myogenin. Several other nuclear proteins, including an alternatively spliced muscle-specific form of α-nascent polypeptide--associated complex, p300/CBP, and RB have also been shown to participate in myogenesis (Missero et al., 1995; Novitch et al., 1996; Yotov and St-Arnaud, 1996; Puri et al., 1997; Sartorelli et al., 1997). Expression of the muscle-specific intermediate filament protein desmin is necessary for myogenesis (Li et al., 1994). ERK6, a muscle-specific mitogen-activated protein kinase, also regulates myogenesis (Lechner et al., 1996). Furthermore, cell surface proteins such as N-CAM, N- and M-cadherin, VLA-4, VCAM-1, and meltrin-α have also been implicated in myogenesis (Menko and Boettiger, 1987; Donalies et al., 1991; Rosen et al., 1992; Peck and Walsh, 1993; Yagami-Hiromasa et al., 1995). Despite this progress, more molecular aspects of myogenesis remain to be investigated.
Using the amino acid sequence of synaptobrevin/VAMP2 to search the database of the expressed sequence tags (ESTs), we have identified a novel member (VAMP5) of the synaptobrevin/VAMP protein family. Interestingly, the expression of VAMP5 is greatly increased during in vitro myogenesis of C2C12 cells. The molecular, biochemical, and cell biological characterizations of VAMP5 are described.
MATERIALS AND METHODS
Materials
Madin–Darby canine kidney strain II (MDCK II) was a generous gift from Dr. Kai Simons (European Molecular Biology Laboratory, Heidelberg, Germany). All other cell lines were obtained from American Type Culture Collection (Rockville, MD). The mouse mRNA multiple tissues Northern filter was obtained from Clontech (Palo Alto, CA). The oligolabeling kit and glutathione–Sepharose 4B beads were purchased from Pharmacia (Upsala, Sweden). Fluorescein isothiocyanate–conjugated goat anti-mouse immunoglobulin G (IgG) and rhodamine-conjugated goat anti-rabbit IgG were purchased from Boehringer Mannheim (Mannheim, Germany). Brefeldin A (BFA) was from Epicentre Technologies (Madison, WI).
cDNA cloning and Sequencing
Mouse EST clones (accession numbers AA050010, AA030509, and AA222692) were generated by the Washington University-Merck EST project and were obtained from the IMAGE consortium via Research Genetics (Huntsville, AL). Clone AA030509 was sequenced completely using the dideoxy chain termination method with the Sequenase II kit (United States Biochemical, Cleveland, OH).
Northern Blot Analysis
A mouse multiple tissue blot of poly(A)+ mRNA (Clontech) was probed with the VAMP5 probe using the procedure as described (Lowe et al., 1996). Total RNA was isolated from myoblasts and myotubes as described previously (Zeng et al., 1994). Total RNA was resolved by 1% agarose gel, transferred to a Hybond-N filter (Amersham, Arlington Heights, IL), and processed for Northern blot as described (Zeng et al., 1994; Lowe et al., 1996).
Expression of Recombinant Proteins in Bacteria
GST-VAMP5 was produced using the pGEX-KG vector (Guan and Dixon, 1991). Briefly, the coding region for residues 1–70 of VAMP5 was retrieved by the PCR using pfu DNA polymerase with oligonucleotides 1 (5′-CTGGATCCATGGCAGGGAAAGAACTGAAG) and 2 (5′-CGTCTAGATTAGTAGACCCGGCACCGGAT). The PCR product was gel purified, digested with restriction enzymes BamHI and XbaI, and then ligated with similarly digested pGEX-KG vector. The ligation reaction was used to transform a competent Escherichia coli M15 strain, and transformants were screened for isopropyl-1-thio-β-d-galactopyranoside–inducible expression of recombinant protein. Two transformants (1 and 2) were expanded for large-scale purification of GST-VAMP5 using a protocol described previously (Lowe et al., 1996).
Preparation of Polyclonal Antibodies
Rabbits were each injected with 500 μg of GST-VAMP5 emulsified in complete Freund’s adjuvant. Booster injections containing a similar amount of antigen emulsified in incomplete Freund’s adjuvant were administered every 2 wk. Rabbits were bled 10 d after the second and subsequent booster injections. Serum was diluted twice with PBS and incubated first with cyanogen bromide–activated Sepharose beads coupled with GST to absorb antibodies against GST. Antibodies against VAMP5 were then affinity purified by incubating with beads immobilized with GST-VAMP5. After extensive washing, specific antibodies were eluted, neutralized, concentrated, and stored at −20°C in 10% glycerol (Lowe et al., 1996; Zhang et al., 1997).
In Vitro Myogenesis
C2C12 cells were grown in DMEM with 20% FBS (growth medium) as myoblasts. When cells reached 50–70% confluency, cell differentiation was initiated by shifting the culture medium to DMEM containing 1–2% horse serum (DM) to trigger myogenesis. Myoblast fusion began 2–3 d after incubation in DM, and maximal myoblast fusion occurred 5 d after incubation in DM. Approximately 40–60% of the cells were in the form of myotubes of varying sizes and morphologies.
Immunoblot Analysis
This was performed as described previously (Lowe et al., 1996; Zhang et al., 1997), except that antibodies against VAMP5 were used at concentrations of 2–5 μg/ml.
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed as described previously (Lowe et al., 1996; Zhang et al., 1997). For the treatment of cells with BFA or nocodazole, myotubes were incubated with BFA (10 μg/ml) or nocodazole (10 μg/ml) for the indicated times, washed twice in PBS with 1 mM CaCl2 and 1 mM MgCl2, and then fixed in 3% paraformaldehyde. Fixed cells were then permeabilized and processed for indirect immunofluorescence microscopy. Confocal microscopy was performed as described previously (Zhang et al., 1997; Tang et al., 1998c) with a Zeiss (Thornwood, NY) Axioplan II microscope equipped with a Bio-Rad (Hercules, CA) MRC1024 confocal scanning laser. Typically, images shown are combinations of 10 optical sections of 0.3 μm apart unless indicated.
Electron Microscopy
C2C12 myotubes were cultured for 8 d and then fixed in 8% paraformaldehyde in 0.1 M phosphate buffer (pH 7.35) for 1 h at room temperature. They were washed with 0.2 M phosphate buffer, scraped from the culture dish, and pelleted at 10,000 rpm in a microfuge. The cells were then resuspended in warm gelatin (10% in phosphate buffer) and repelleted at maximum speed in the microfuge. After cooling, the gelatin-embedded cells were infiltrated with polyvinyl pyrrolidone–sucrose overnight at 4°C and then processed for frozen sectioning as described previously (Parton et al., 1997). Ultrathin frozen sections (60–80 nm) were labeled, viewed (Jeol 1010, Centre for Microscopy and Microanalysis, University of Queensland) according to published techniques (Parton et al., 1997), except that to increase the signal the first antibody (affinity-purified anti-VAMP5) was followed by a swine anti-rabbit second antibody before incubation with 10 nm protein A-gold (University of Utrecht, Utrecht, The Netherlands)
Differential Extraction of Total Membranes
The total membrane of myotube was prepared as described (Lowe et al., 1996). Briefly, cells were sonicated in PBS and then centrifuged at 2000 × g to remove the unbroken cells and the nuclei. The supernatant was centrifuged at 10,000 × g for 1 h at 4°C to yield a total membrane pellet. The membrane was extracted on ice for 1 h in 100 μl of PBS, 1 M KCl, 0.15 M sodium bicarbonate (pH 11.5), 2.5 M urea, 1% Triton X-100, or 1% deoxycholate (DOC) and then centrifuged at 100,000 × g for 1 h at 4°C. The supernatants were transferred to another tube, and the pellets were resuspended in 100 μl of 1× SDS sample buffer. Aliquots (20 μl) from both the supernatants as well as the pellets were analyzed by immunoblot analysis to detect VAMP5.
Epitope Tagging and Transfection
PCR reactions with oligos 3 (5′-GCGAATTCACCATGGAGCAGAAGCTGATCTCCGAGGAGGACCTCGCAGGGAAAGAACTGAAGCAATG) and 4 (5′-CTGGATCCTCTAGACTATGGTTTACTACTGTCCC) or with oligos 5 (5′-GCGAATTCACCATGGCTTACCCATACGATGTTCCAGATTACGCTGCAGGGAAAGAACTGAAGCAATG) and 4 were performed to introduce myc and hemagglutinin (HA) epitope at the N terminus of VAMP5. The PCR fragments were cut with EcoRI and BamHI, inserted into the expression vector pCI-neo (Promega, Madison, WI), and then stably transfected into C2C12 cells as described (Lowe et al., 1997).
RESULTS
VAMP5 as a Novel Member of the Synaptobrevin/VAMP Protein Family
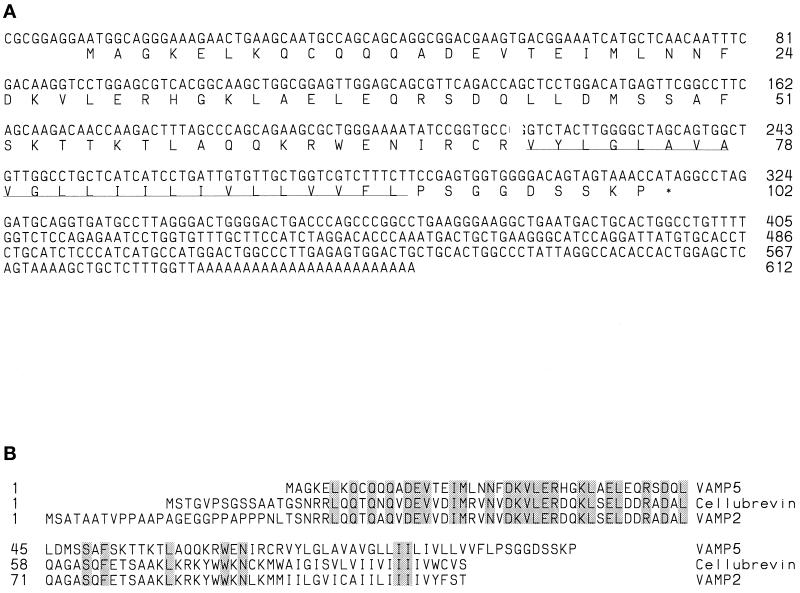
Three homologous mouse EST clones (accession numbers AA050010, AA030509, and AA222692) were recovered through EST database searches (Altschul et al., 1990) with the amino acid sequence of mouse synaptobrevin/VAMP-2. These three EST clones represent the same protein based on the comparison of available EST sequences and partial sequences obtained by resequencing the cDNA clones. EST clone AA030509 was sequenced completely. The existence of EST clone AA222692 was also noticed by Bock and Scheller (1997), and they have referred to the corresponding protein as VAMP5. To avoid confusion of nomenclature for this protein, we have adopted the name VAMP5 for this protein. The nucleotide and deduced amino acid sequences of mouse VAMP5 are shown in Figure 1A. The 102–amino acid sequence of VAMP5 contains a 23-residue hydrophobic domain (residues 71–93) near the carboxyl terminus that could function as a carboxyl-terminal membrane anchor. The N-terminal 70-residue sequence (residues 1–70) is predicted to be oriented on the cytoplasmic side of the membrane, whereas the carboxyl-terminal nine-residue sequence (residues 94–102) is predicted to be oriented in the lumen of intracellular membrane compartments or exposed to the extracellular side of the plasma membrane. The putative N-terminal cytoplasmic domain can potentially form coiled-coil structures, a prediction based on analysis using the COILS 2.1 program (http://www.isrec.isb-sib.ch/software/COILS_form.html). The alignment of amino acid sequences of mouse VAMP5, cellubrevin, and synaptobrevin/VAMP-2 is shown in Figure 1B. VAMP5 exhibits an overall 33% identity with cellubrevin and synaptobrevin/VAMP2. As shown, the amino acid sequences of VAMP5, cellubrevin, and synaptobrevin/VAMP2 can be aligned without introducing any gaps. These results establish that VAMP5 is indeed a novel member of the synaptobrevin/VAMP protein family.
Figure 1.
(A) Nucleotide and the derived amino acid sequence of mouse VAMP5. The C-terminal hydrophobic region is underlined. (B) Alignment of amino acid sequences of mouse VAMP5, cellubrevin, and VAMP2/synaptobrevin 2. Residues identical in the three proteins are shaded.
VAMP5 Transcript Is Preferentially Expressed in the Muscle and Heart
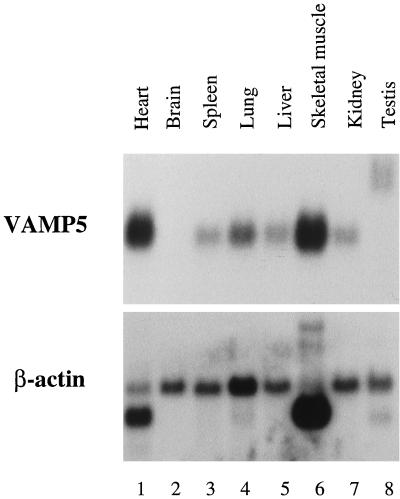
To initiate molecular characterization of VAMP5, we have examined its mRNA levels in several mouse tissues. A mouse multiple-tissue mRNA blot (Clontech) was probed with radiolabeled VAMP5 cDNA probe. As shown in Figure 2A, a major mRNA species of ∼700 bp was detected. mRNA for VAMP5 was present at the highest levels in the skeletal muscle and heart. Much lower levels were detected in the spleen, lung, liver, kidney, and testis. VAMP5 mRNA was not detected in the brain. Interestingly, VAMP5 mRNA in the testis has a larger size (∼1.4–1.5 kbp) than that in other tissues. Although the molecular basis for this size difference remains to be examined, the larger size of the transcript in the testis could be derived from alternative splicing and/or use of a different downstream polyadenylation signal. The levels of β-actin mRNA in the various tissues of the same blot are shown in Figure 2B.
Figure 2.
Northern blot analysis of VAMP5 transcript. A mouse multiple-tissue blot containing 2 μg of poly(A)+ mRNA from the indicated tissues was probed with 32P-labeled VAMP5 cDNA probe (top panel) and then subsequently with 32P-labeled β-actin probe (bottom panel). Highest levels of VAMP5 transcript were detected in the heart and the skeletal muscle.
VAMP5 mRNA Levels Are Increased during In Vitro Myogenesis of C2C12 Cells
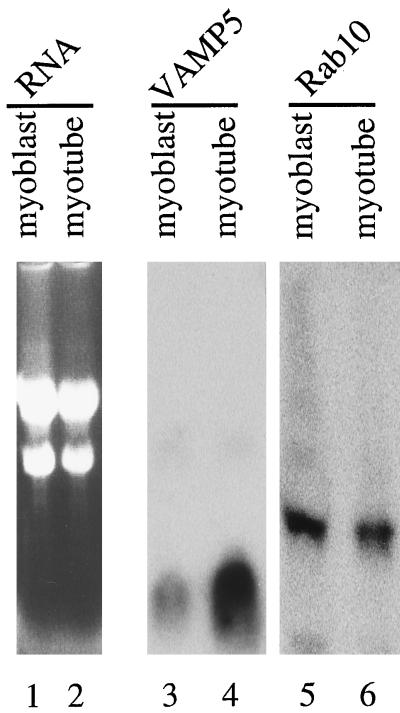
The preferential expression of VAMP5 in the skeletal muscle and heart indicates that VAMP5 may be associated with a function that is more prominent in these tissues. To explore this further, we have examined the levels of VAMP5 mRNA during in vitro myogenesis of mouse C2C12 cells. C2C12 cells grow as myoblasts in growth medium and can be induced to differentiate into myotubes in differentiation medium. Total RNA was isolated from myoblasts and myotubes and analyzed by Northern blotting (Figure 3). Lanes 1 and 2 show the loading of the RNA stained with ethidium bromide, whereas lanes 3 and 4 show the results of after hybridization with the VAMP5 cDNA probe. It is obvious that VAMP5 mRNA is much more abundant in the myotubes (Figure 3, lanes 2 and 4) compared with myoblasts (Figure 3, lanes 1 and 3). In marked contrast, the mRNA for mouse Rab10, a small GTPase implicated in post-Golgi trafficking (Chen et al., 1993), is not increased during in vitro myogenesis (Figure 3, lanes 5 and 6). It is estimated that VAMP5 mRNA levels are increased by ∼8- to 10-fold during in vitro myogenesis.
Figure 3.
Increase in VAMP5 transcript during in vitro myogenesis. Thirty micrograms of total RNA isolated from myoblasts (lanes 1, 3, and 5) and 15 μg of total RNA isolated from myotubes (lanes 2, 4, and 6) were analyzed by agarose gel electrophoresis. The RNAs were stained with ethidium bromide and photographed (lanes 1 and 2) or transferred to a filter and probed with 32P-labeled VAMP5 probe (lanes 3 and 4) or Rab10 probe (lanes 5 and 6).
Characterization of VAMP5-specific Antibodies
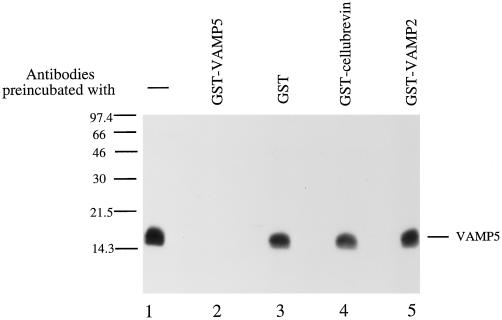
To facilitate biochemical and cell biological characterizations of VAMP5, we have raised rabbit antibodies against VAMP5. The cytoplasmic region (residues 1–70) of VAMP5 was expressed in bacteria as a fusion protein with GST (GST-VAMP5), and the purified GST-VAMP5 was used to immunize rabbits. Rabbit antisera were first absorbed with immobilized GST to remove antibodies against the GST portion of the fusion protein. Antibodies specific for VAMP5 were then affinity purified with immobilized GST-VAMP5. Immunoblot analysis (Figure 4) demonstrates that the antibodies prepared in this way recognize specifically a 16-kDa polypeptide in total extract of myotubes (Figure 4, lane 1). Detection of this protein is abolished by preincubation of the antibodies with GST-VAMP5 (Figure 4, lane 2) but not with GST (Figure 4, lane 3), GST-cellubrevin (Figure 4, lane 4), or GST-VAMP2 (Figure 4, lane 5). These results establish that the affinity-purified antibodies are specific for VAMP5. Although the apparent size of VAMP5 (16 kDa) is ∼4.5 kDa larger than the predicted size (11.5 kDa), a similar difference between the apparent size (18 kDa) and the predicted size (13 kDa) for synaptobrevin1/VAMP1 has also been observed (Baumert et al., 1989). We thus conclude that the 16-kDa polypeptide is VAMP5.
Figure 4.
Characterization of VAMP5 antibodies. Total cellular extracts of myotubes were resolved by SDS-PAGE and transferred to a filter. The blot was probed with affinity-purified rabbit antibodies against VAMP5, and a 16-kDa protein was specifically detected (lane 1). The detection of this protein is abolished by preincubation of antibodies with recombinant GST-VAMP5 (lane 2) but not by GST (lane 3), GST-cellubrevin (lane 4), or GST-VAMP2 (lane 5).
VAMP5 Levels Are Also Induced during In Vitro Myogenesis
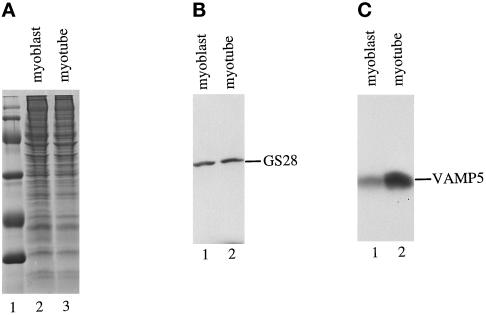
Because the VAMP5 transcript level is increased during myogenesis, we examined whether the protein level of VAMP5 is similarly induced during myogenesis. Total detergent extracts of myoblasts and myotubes were analyzed by immunoblot analysis to detect VAMP5. As shown in Figure 5, the amount of VAMP5 is clearly increased during myogenesis. The level of VAMP5 in myotubes (Figure 5C, lane 2) is approximately sixfold higher than in myoblasts (Figure 5C, lane 1), an estimation based on a series of dilutions of myotube extract compared with a fixed amount of myoblast extract. As a control, GS28 (also referred to as GOS-28), a cis-Golgi SNARE involved in protein transport from the endoplasmic reticulum to the Golgi and/or intra-Golgi transport (Subramaniam et al., 1995, 1996; Nagahama et al., 1996), is present at similar levels in both myoblasts and myotubes (Figure 5B).
Figure 5.
Increase of VAMP5 protein during myogenesis. One hundred micrograms of total protein extracts derived from growing myoblasts (A, lane 2; B, lane 1; C, lane 1) and myotubes after growing in differentiation medium for 5 d (A, lane 3; B, lane 2; C, lane 2) were resolved by SDS-PAGE and stained with Coommasie blue (A) or processed for immunoblot to detect Golgi SNARE GS28 (B) or VAMP5 (C). Although GS28 is present at comparable levels in both myoblasts and myotubes, VAMP5 is present at much higher levels in myotubes.
VAMP5 Is an Integral Membrane Protein
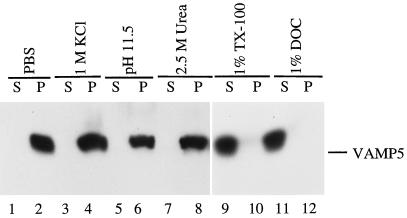
The deduced amino acid sequence of VAMP5 suggests that it is an integral membrane protein. To biochemically verify this point, we prepared a total membrane fraction of myotubes and subjected it to different extraction conditions as detailed in Figure 6. The extracted supernatants (S) and the pellets (P) were analyzed for VAMP5 by immunoblotting. As shown, VAMP5 was not extracted by PBS, 1 M KCl, 2.5 M urea, or 0.15 M sodium bicarbonate (pH 11.5) (Figure 6, lanes 1–8) but was effectively extracted by detergents such as 1% Triton X-100 and 1% DOC (Figure 6, lanes 9–12), establishing that VAMP5 is indeed an integral membrane protein.
Figure 6.
VAMP5 is an integral membrane protein. Total membranes derived from myotubes were extracted by different reagents as indicated. The extracts (S) and the membrane pellets (P) were then analyzed by immunoblot to detect VAMP5. As shown, VAMP5 is not extracted by PBS (lanes 1 and 2), 1 M KCl (lanes 3 and 4), 150 mM sodium bicarbonate (pH 11.5) (lanes 5 and 6), or 2.5 M urea (lanes 7 and 8) but is extracted effectively by detergents such as 1% Triton X-100 (lanes 9 and 10) and 1% DOC (lanes 11 and 12).
Association of VAMP5 with the Plasma Membrane and Intracellular Vesicular Structures in Myotubes
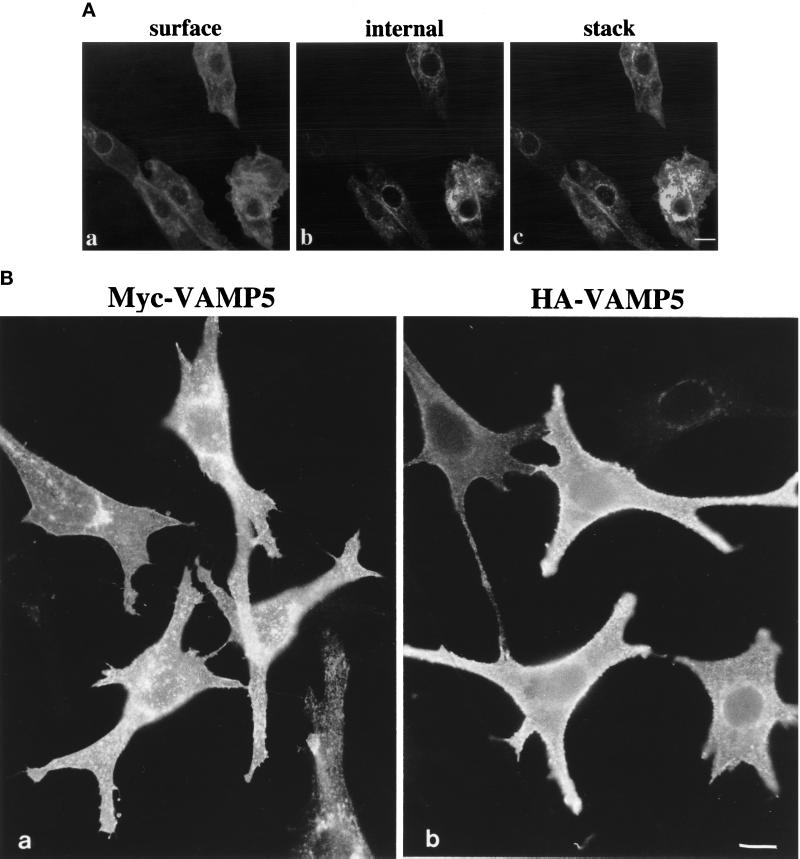
By indirect immunofluorescence microscopy, the affinity-purified antibodies against VAMP5 failed to reveal any specific labeling in several cell lines such as MDCK, NRK, A431, Hela, CHO, and growing C2C12 cells. To gain insight into its subcellular localization, we examined the subcellular localization of VAMP5 in differentiated C2C12 cells. After growing in differentiation medium for 5–6 d, ∼40–60% of cells are in the form of fused multinuclear myotubes with the rest remaining as unfused myoblasts. Although only basal levels of labeling were detected in the majority of the unfused myoblasts, some unfused myoblasts have intense labeling on the cell surface (Figure 7A, a) and intracellular vesicular structures (7A, b), as is also obvious from the combined optical sections (7A, c). Strong labeling was detected in every myotube (Figures 8–11). Prominent labeling could be found on the plasma membrane and intracellular vesicular structures. These results not only demonstrate again that VAMP5 level is greatly enhanced during myogenesis but also that VAMP5 is associated with the plasma membrane and intracellular vesicular structures.
Figure 7.
(A) Labeling of VAMP5 in few myoblasts in differentiated C2C12 cultures by indirect immunofluorescence microscopy. Cells were fixed, permeabilized, and incubated with affinity-purified rabbit antibodies against VAMP5 followed by FITC-conjugated anti-rabbit IgG. The cells were mounted, and images captured with the Bio-Rad MRC1024 confocal system. Although the majority of the unfused myoblasts did not exhibit any specific labeling, a small fraction of unfused myoblasts had strong labeling on the cell surface (A, a) and intracellular vesicular structures (A, b) when viewed at the cell surface and internal focal planes, respectively. Shown in c (A) is the combined image of 0.3-μm optical sections, and it is again obvious that VAMP5 is associated with the plasma membrane and intracellular vesicular structures. (B) Epitope-tagged versions of VAMP5 are targeted to the cell surface. Pooled transfectants of C2C12 cells stably transfected with expression vector expressing myc-tagged VAMP5 (a) or HA-tagged VAMP5 (b) were fixed and labeled with monoclonal antibody against myc or HA followed by FITC-conjugated anti-mouse IgG. Cells were viewed and photographed. Bars, 10 μm.
Figure 8.
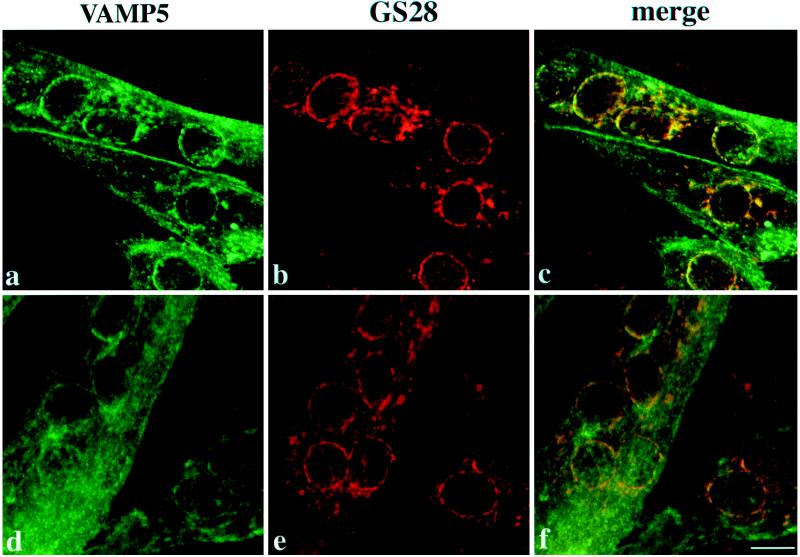
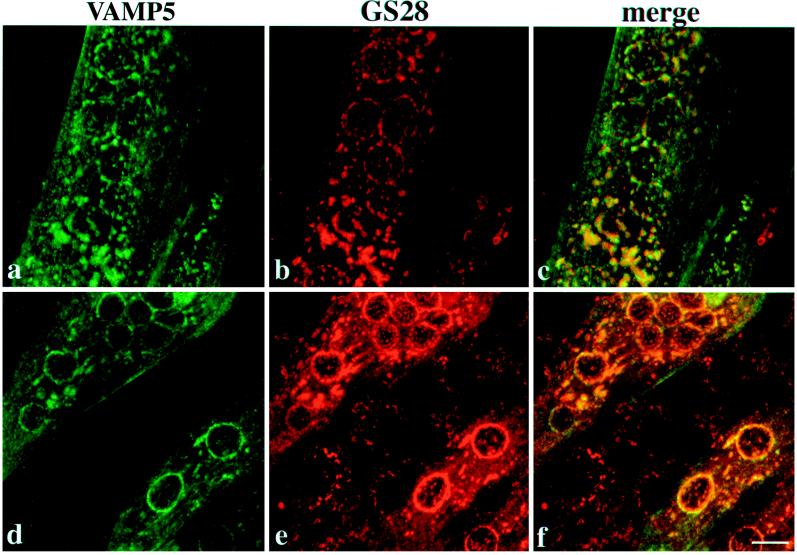
Differentiated C2C12 cells were double-labeled with rabbit antibodies against VAMP5 and a mouse monoclonal antibody against GS28 and then with FITC-conjugated anti-rabbit IgG and rhodamine-conjugated anti-mouse IgG. Cells were viewed with confocal microscopy, and combined images are shown. In myotubes, both surface and intracellular labelings are detected, and VAMP5 is thus associated with the plasma membrane and intracellular vesicular structures of myotubes. The broken-ring labeling of VAMP5 around the nuclei overlaps well with that of GS28, as does some of the peripheral labeling. However, the labeling on the plasma membrane and the majority of peripheral vesicular structures labeled by VAMP5 are devoid of GS28 As shown, most unfused myoblasts (except for the one on the right bottom corners of a–c) do not exhibit specific labeling of VAMP5, although their Golgi apparatuses are labeled by the GS28 monoclonal antibody. Bar, 10 μm.
Figure 11.
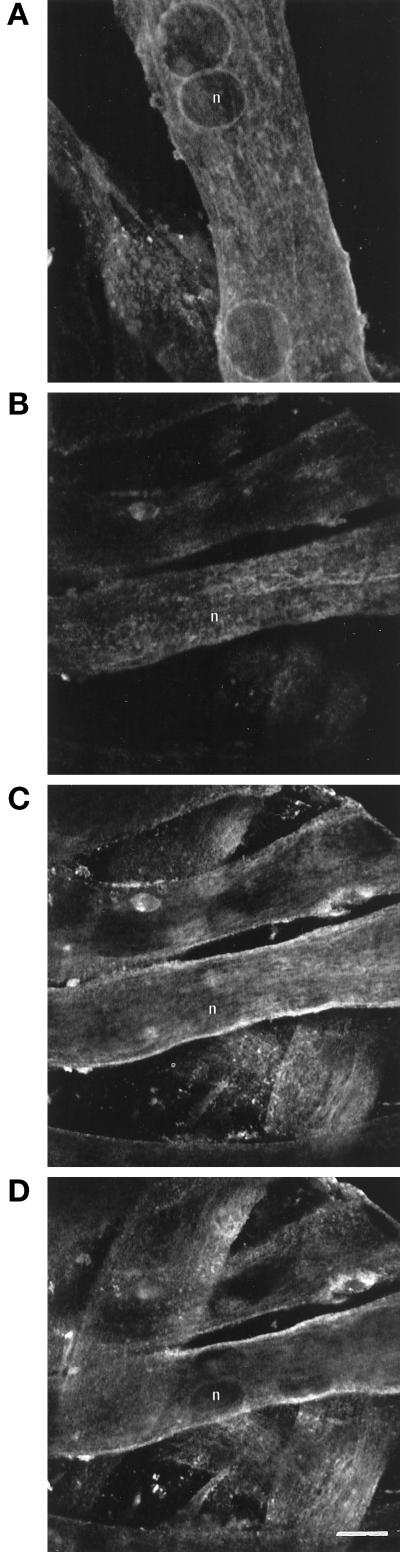
Immunolabeling of VAMP5 in young and mature C2C12 myotube cultures. C2C12 cells were grown in culture snd studied at either day 3 (a) or day 9 (b–d) after differentiation. Cells were fixed and labeled with anti-VAMP5 antibodies. In young cultures, both intracellular and surface labelings were evident. In mature cultures, surface labeling became more predominant. Panels b–d represent sequential serial sections using a confocal laser microscope through the same field of a mature culture and illustrate the relative absence of intracellular labeling compared with that observed in panel a. Representative nuclei in both preparations are indicated (n). Bar, 10 μm.
Two types of vesicular structures were observed in myotubes, one being associated with broken ring-like structures around the nuclei, whereas the other was distributed peripherally. To gain additional insight into the intracellular vesicular structures, we performed double labeling with rabbit antibodies against VAMP5 and a mouse monoclonal antibody against GS28 (Subramaniam et al., 1995, 1996). As shown, the broken ring-like structures of VAMP5 and GS28 colocalized (Figure 8). Colocalization of some of the peripheral structures of VAMP5 and GS28 was also seen. However, the majority of peripheral labeling of VAMP5 as well as the labeling on the plasma membrane was unique for VAMP5. Also obvious in Figure 8 is that the majority of unfused myoblasts (with the exception of one unfused myoblast in the bottom right corner of a–c) did not exhibit detectable labeling for VAMP5, although GS28 labeling was observed.
Nocodazole is known to fragment the Golgi apparatus (Yang and Storrie, 1998), and we treated C2C12 cells after growing in differentiation medium for 5–6 d with 10 μg/ml nocodazole for 1 h. The cells were then processed for double labeling to detect VAMP5 and GS28 (Figure 9). The Golgi apparatus marked by GS28 in unfused myoblasts (between the two myotubes) was clearly fragmented (Figure 9, e and f). Interestingly, the Golgi apparatus marked by GS28 in myotubes was less fragmented. Under this condition, the perinuclear labeling of VAMP5 seems to be segregated significantly from that of GS28, although their labeling patterns still show considerable overlap (Figure 9, c and f). These results indicate that VAMP5 and GS28 may not reside in the same compartment, although they appear to be colocalized under normal conditions.
Figure 9.
Differentiated C2C12 cells were incubated with 10 μg/ml nocodazole for 60 min and then analyzed by indirect immunofluorescence microscopy to detect VAMP5 and GS28. The top and bottom panels represent views from different myotubes. Note that in the bottom panel, unfused myoblasts are labeled by anti-GS28 antibody (e and f) but are negative for VAMP5 labeling (d and f). Although the Golgi apparatus marked by GS28 in unfused myoblasts is clearly fragmented, the Golgi apparatus marked by GS28 in myotubes is less fragmented. Under this condition, the Golgi-like labeling of VAMP5 is significantly segregated from that of GS28. Bar, 10 μm.
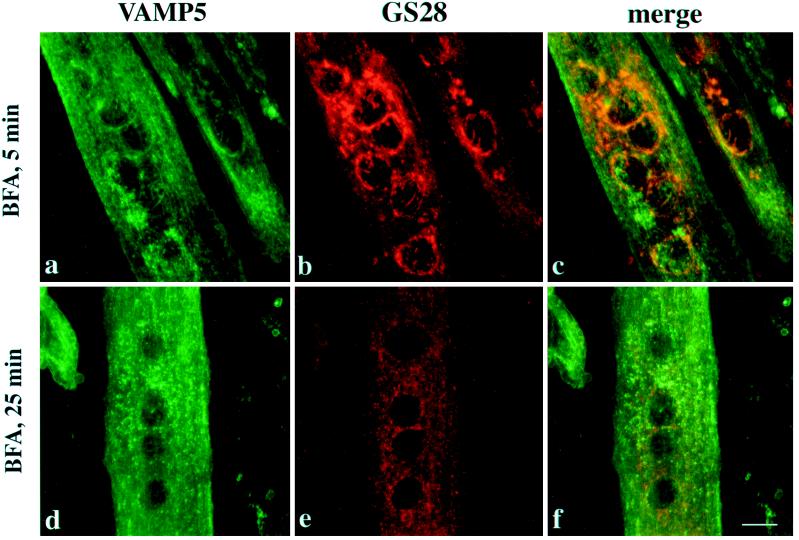
BFA has dramatic effects on the secretory and endocytotic pathway (Klausner et al., 1992). When myotubes were treated with 10 μg/ml BFA for 5 min, the majority of the Golgi-like labeling of VAMP5 was seen to be redistributed (Figure 10a), whereas the Golgi labeling of GS28 remained prominent (Figure 10b). After a 25-min treatment with BFA, both VAMP5 (Figure 10d) and GS28 (Figure 10e) were distributed into small vesicle-like structures. Importantly, VAMP5 and GS28 were associated with distinct structures that no longer colocalized (Figure 10f). This demonstrates that VAMP5 and GS28 are redistributed into distinct structures by BFA treatment.
Figure 10.
Differentiated C2C12 cells were incubated with BFA for 5 or 25 min and then processed for indirect immunofluorescence microscopy to detect VAMP5 and GS28. After a 5-min treatment with BFA, the perinuclear Golgi-like labeling for VAMP5 is essentially dispersed (a and c), although the perinuclear Golgi labeling for GS28 remains essentially intact (b and c), suggesting that structures marked by VAMP5 are more quickly affected by BFA. After 25 min of BFA treatment, both VAMP5 (d) and GS28 (e) are redistributed into fine dotted structures. However, the majority of VAMP5-positive structures do not colocalize with that of GS28, suggesting that VAMP5 and GS28 are segregated into distinct structures. Bar, 10 μm.
We have examined the expression and localization of VAMP5 during different times of differentiation. The results are consistent with the above in that every myotube exhibits strong labeling, whereas only a fraction of myoblasts has strong labeling on day 2 and onward after differentiation. However, it was observed that both surface and intracellular labeling of VAMP5 were evident in young myotubes (Figure 11a), whereas the surface labeling for VAMP5 became more predominant in more matured myotubes (Figure 11b–d).
Localization of Epitope-tagged Versions of VAMP5 in Transfected C2C12 Myoblasts
The subcellular localization of VAMP5 was also examined by stable expression of myc epitope- as well as HA epitope-tagged versions of VAMP5 in C2C12 cells. As revealed by indirect immunofluorescence microscopy (Figure 7B), both the myc-tagged version (myc-VAMP5) as well as the HA-tagged version (HA-VAMP5) are preferentially associated with the plasma membrane with some intracellular vesicular labeling. Labeling of myc-VAMP5 and HA-VAMP5 in myotubes was similar to that observed for endogenous VAMP5. These results further confirm the conclusion that VAMP5 is associated with the plasma membrane and intracellular vesicular structures.
Immunogold Labeling of VAMP5 in C2C12 Myotubes
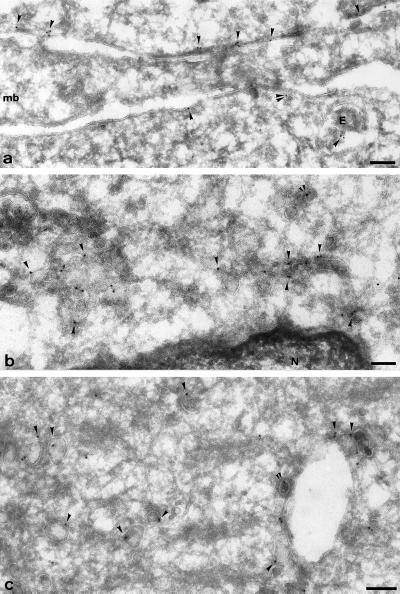
The distribution of VAMP5 was further examined at the ultrastructural level on ultrathin frozen sections of differentiated C2C12 cells. Specific labeling was observed on the plasma membrane and internal membrane of myotubes (Figure 12). Undifferentiated myoblasts, which are also present in the cultures after differentiation, had very low labeling for VAMP5 (e.g., Figure 12a, cell marked mb), consistent with the above results and showing the specificity of the immunogold labeling. Labeling of both the plasma membrane and intracellular structures were observed in myotubes. Plasma membrane labeling was patchy but not particularly concentrated on identifiable features. Further work will be required to ascertain whether this represents a random distribution over the entire surface or some concentration in certain structures. The specific labeling of intracellular tubular–vesicular structures may represent the endosomal compartment (Figure 12, a and c), as well as uncoated vesicles and tubules in both cell periphery (Figure 12c) and in the perinuclear area (Figure 12b). These results further establish that VAMP5 is associated with the plasma membrane and intracellular structures of myotubes.
Figure 12.
Ultrastructural localization of VAMP5. Ultrathin frozen sections of differentiated C2C12 cells were labeled with anti-VAMP5 antibodies followed by swine anti-rabbit antibodies and then 10 nm protein A-gold. Panel a shows plasma membrane regions of three closely apposed cells; a region of myoblast (mb) is unlabeled, whereas the neighboring myotubes (above and below) show significant labeling close to the plasma membrane (arrowheads). Note the labeling on a small vesicle (double arrowheads) and on a multivesicular endosome (E). Panel b shows the juxtanuclear area of a myotube; labeling is concentrated on uncoated vesicular and tubular profiles. The ER surrounding the nucleus (N) is unlabeled. Panel c shows characteristic labeling of large uncoated vesicles (arrowheads), some of which are associated with putative endosomal structure. The double arrowheads in b and c indicate a labeled clathrin coated buds. Bars, 100 nm.
DISCUSSION
The skeletal muscle and the heart are unique tissues because they are composed mainly of multinuclear myotubes derived from the fusion of myoblasts. Two specific membrane trafficking processes are associated with these two tissues. The first one is the fusion of myoblasts into myotubes (Lassar et al., 1994; Tam et al., 1995; Molkentin and Olson, 1996; Rawls and Olson, 1997; Walsh and Perlman, 1997). Although much remains to be investigated, a recent study has shown that there exists a unique class of small vesicles that are associated with the myoblast fusion process (Doberstein et al., 1997). Second, like adipocytes, myotubes are the major cells expressing the glucose transporter 4 (GLUT4), which is normally sequestered intracellularly in storage compartments (James and Piper, 1994). Upon stimulation by insulin, GLUT4 is transported to the plasma membrane to mediate glucose uptake. It remains unclear whether there exist specific SNAREs that are preferentially expressed in the skeletal muscle and the heart to facilitate these two physiologically important trafficking events. A recent study aiming to identify novel members of the synaptobrevin/VAMP family in the skeletal muscle using the PCR approach had revealed the expression of synaptobrevin 1 and 2 in the skeletal muscle but failed to detect any novel members of this family that are preferentially expressed in the skeletal muscle (Ralston et al., 1994).
In the present study, we have identified a novel member (VAMP5) of the synaptobrevin/VAMP family. We have provided evidence that the expression of VAMP5 is associated with myogenesis. First, the mRNA for VAMP5 is expressed at the highest levels in the skeletal muscle and the heart among the eight tissues examined by Northern blot analysis, suggesting that the VAMP5 transcript is preferentially expressed in the skeletal muscle and the heart. Second, during in vitro myogenesis of C2C12 cells, the mRNA for VAMP5 is increased ∼8- to 10-fold in the myotubes compared with myoblasts. Because only ∼40–60% of the C2C12 cells are fused into myotubes in our experiments, the mRNA extracted from myotubes also contains those derived from the unfused myoblasts. We estimate that only half of the mRNA is derived from authentic myotubes and that the mRNA for VAMP5 may actually be increased by a factor of ∼15- to 20-fold in the myotubes. Third, immunoblot analysis of total cellular extracts of myotubes and myoblasts revealed that VAMP5 is increased approximately sixfold in the myotubes compared with the myoblasts. Again, because myotube extracts also contain those derived from unfused myoblasts, VAMP5 levels may be increased by a factor of 10 in the myotubes. Finally, indirect immunofluorescence and immunogold microscopy using affinity-purified antibodies against VAMP5 did not reveal specific labeling in myoblasts. In cells grown in differentiation medium, essentially all the myotubes exhibit strong labeling of the plasma membrane and intracellular vesicular structures, whereas the majority of the unfused myoblasts display only background labeling. Only a small fraction of unfused myoblasts contain strong VAMP5 labeling in the plasma membrane and intracellular structures. These results are consistent with the notion that the VAMP5 protein level is greatly increased in the myotubes. The preferential expression of VAMP5 mRNA in the skeletal muscle and the heart as well as the increased levels of VAMP5 mRNA and protein during in vitro myogenesis are further sustained by the observation that no specific labeling for VAMP5 is detected in diverse cell lines such as NRK, MDCK, A431, and Hela cells.
The results of indirect immunofluorescence microscopy not only support the conclusion that the level of VAMP5 is greatly increased during myogenesis but also reveal the subcellular localization of VAMP5. VAMP5 is clearly associated with the plasma membrane and intracellular vesicular structures. The association of VAMP5 with the plasma membrane is further supported by the observation that both myc and HA epitope-tagged versions of VAMP5 are targeted to the cell surface in stably transfected cells. The intracellular vesicular structures are located both peripherally as well as in the Golgi region marked by Golgi SNARE GS28 (Subramaniam et al., 1995, 1996; Nagahama et al., 1996). In cells treated with nocodazole, the Golgi-like labeling of VAMP5 is segregated significantly from that of GS28. In cells treated with BFA, VAMP5 is redistributed much faster than GS28 and the Golgi-like labeling of VAMP5 is essentially redistributed after 5-min treatment with BFA, whereas the Golgi labeling of GS28 remains essentially intact. Furthermore, VAMP5 and GS28 are seen to be redistributed into distinct smaller vesicular structures after treatment with BFA for 25 min. Because GS28 behaves like a protein of the Golgi stack and the intermediate compartment upon treatment with BFA (Subramaniam et al., 1995), the different response of VAMP5 to BFA suggests that VAMP5 is not associated with the cis-Golgi or the Golgi stack. The most likely possibility is that VAMP5 is associated with structures associated with the trans region of the Golgi apparatus. The distribution of VAMP5 in the plasma membrane and intracellular structures was further established by immuno-gold labeling of differentiated C2C12 cells.
The relationship between the surface and the intracellular pools of VAMP5 in myotubes remains unclear. Two possibilities exist. One is that the intracellular pool represents those VAMP5 molecules en route to the plasma membrane. Alternatively, the surface and the intracellular pools are in dynamic equilibrium and that VAMP5 cycles between these two locations. Future experiments are needed to distinguish between these two possibilities. Although the functional aspects of VAMP5 remain to be investigated, the preferential expression of VAMP5 in the skeletal muscle and heart, the increased expression of VAMP5 during in vitro myogenesis, and the observation that VAMP5 is undetectable by indirect immunofluorescence microscopy in diverse cell lines suggest that VAMP5 may participate in a trafficking event that is associated with myogenesis in these tissues. Whether VAMP5 plays a role in myoblast fusion and/or GLUT4 trafficking remains to be experimentally examined.
ACKNOWLEDGMENTS
We thank Drs. T.C. Südhof for the cDNA clone of cellubrevin and Tony Ting for plasmid expressing GST-VAMP2. We thank Dr. P. Singh for reading the manuscript and Dr. Y.H. Tan for his continuous support.
REFERENCES
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baumert M, Maycox PR, Navone F, De Camilli P, Jahn R. Synaptobrevin: an integral membrane protein of 18,000 daltons present in small synaptic vesicles of rat brain. EMBO J. 1989;8:379–384. doi: 10.1002/j.1460-2075.1989.tb03388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Garcia-Arraras JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH. The syntaxin family of vesicular transport receptors. Cell. 1993;74:863–873. doi: 10.1016/0092-8674(93)90466-4. [DOI] [PubMed] [Google Scholar]
- Bock JB, Klumperman J, Davanger S, Scheller RH. Syntaxin 6 functions in trans-Golgi network vesicle trafficking. Mol Biol Cell. 1997;8:1261–1271. doi: 10.1091/mbc.8.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock JB, Lin RC, Scheller RH. A new syntaxin family member implicated in targeting of intracellular transport vesicle. J Biol Chem. 1996;271:17961–17965. doi: 10.1074/jbc.271.30.17961. [DOI] [PubMed] [Google Scholar]
- Bock JB, Scheller RH. Protein transport. A fusion of new ideas. Nature. 1997;387:133–135. doi: 10.1038/387133a0. [DOI] [PubMed] [Google Scholar]
- Chen YT, Holcomb C, Moore HP. Expression and localization of two low molecular weight GTP-binding proteins, Rab8 and Rab10, by epitope tag. Proc Natl Acad Sci USA. 1993;90:6508–6512. doi: 10.1073/pnas.90.14.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein SK, Fetter RD, Mehta AY, Goodman CS. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donalies M, Cramer M, Ringwald M, Starzinski-Powitz AE. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci USA. 1991;88:8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro-Novick S, Jahn R. Vesicle fusion from yeast to man. Nature. 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- Guan K, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hay JC, Chao DS, Kuo CS, Scheller RH. Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell. 1997;89:149–158. doi: 10.1016/s0092-8674(00)80191-9. [DOI] [PubMed] [Google Scholar]
- Hay JC, Harald H, Scheller RH. Mammalian vesicle trafficking proteins of the endoplasmic reticulum and Golgi apparatus. J Biol Chem. 1996;271:5671–5679. doi: 10.1074/jbc.271.10.5671. [DOI] [PubMed] [Google Scholar]
- Hong W. Protein Trafficking along the Exocytotic Pathway. Austin, TX: R.G. Landes; 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DE, Piper RC. Insulin resistance, diabetes, and the insulin-regulated trafficking of GLUT-4. J Cell Biol. 1994;126:1123–1126. doi: 10.1083/jcb.126.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar AB, Skapek SX, Novitch B. Regulatory mechanisms that coordinate skeletal muscle differentiation and cell cycle withdrawal. Curr Opin Cell Biol. 1994;6:788–794. doi: 10.1016/0955-0674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Lechner C, Zahalka MA, Giot JF, Moller NP, Ullrich A. ERK6, a mitogen-activated protein kinase involved in C2C12 myoblast differentiation. Proc Natl Acad Sci USA. 1996;93:4355–4359. doi: 10.1073/pnas.93.9.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Choudhary SK, Milner DJ, Munir MI, Kuisk IR, Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol. 1994;124:827–841. doi: 10.1083/jcb.124.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe SL, Peter F, Subramaniam VN, Wong SH, Hong W. A Golgi SNARE (GS27) involved in transport from the cis/medial- to the trans-Golgi/TGN. Nature. 1997;389:881–884. doi: 10.1038/39923. [DOI] [PubMed] [Google Scholar]
- Lowe SL, Wong SH, Hong W. The mammalian ARF-like protein 1 (Arl1) is associated with the Golgi complex. J Cell Sci. 1996;109:209–220. doi: 10.1242/jcs.109.1.209. [DOI] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Südhof TC. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Sollner TH. Ykt6p, a prenylated SNARE essential for endoplasmic reticulum-Golgi transport. J Biol Chem. 1997;272:17776–17783. doi: 10.1074/jbc.272.28.17776. [DOI] [PubMed] [Google Scholar]
- Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation. Proc Natl Acad Sci USA. 1995;92:5451–5455. doi: 10.1073/pnas.92.12.5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Genet Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Nagahama M, Orci L, Ravazzola M, Amherdt M, Lacomis L, Tempst P, Rothman JE, Söllner TH. A v-SNARE implicated in intra-Golgi transport. J Cell Biol. 1996;133:507–516. doi: 10.1083/jcb.133.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Mulligan GJ, Jacks T, Lassar AB. Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle. J Cell Biol. 1996;135:441–456. doi: 10.1083/jcb.135.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I, Orci L, Ravazzola M, Erdjument-Bromage H, Amherdt M, Tempst P, Sollner TH, Rothman JE. ERS-24, a mammalian v-SNARE implicated in vesicle traffic between the ER and the Golgi. J Cell Biol. 1997;137:1017–1028. doi: 10.1083/jcb.137.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE. Intracellular aspects of the processing of protein synthesis. Science. 1975;189:347–354. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Parton RG, Way M, Zorzi N, Stang E. Caveolin-3 associates with developing T-tubules during muscle differentiation. J Cell Biol. 1997;136:137–154. doi: 10.1083/jcb.136.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck D, Walsh FS. Differential effects of over-expressed neural cell adhesion molecule isoforms on myoblast fusion. J Cell Biol. 1993;123:1587–1595. doi: 10.1083/jcb.123.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Pryer NK, Wuestehube LJ, Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Puri PL, et al. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- Ralston E, Beushausen S, Ploug T. Expression of the synaptic vesicle proteins VAMPs/synaptobrevins 1 and 2 in non-neural tissues. J Biol Chem. 1994;269:15403–15406. [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA. Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem. 1996;271:13300–13303. doi: 10.1074/jbc.271.23.13300. [DOI] [PubMed] [Google Scholar]
- Rawls A, Olson EN. MyoD meets its maker. Cell. 1997;89:5–8. doi: 10.1016/s0092-8674(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanism of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr Biol. 1994;4:220–233. doi: 10.1016/s0960-9822(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Huang J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1532. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Scheller RH. Membrane trafficking in the presynaptic nerve terminal. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Krijnse-Locker J, Tang BL, Ericsson M, Yusoff ARbM, Griffiths G, Hong W. Monoclonal antibody HFD9 identifies a novel 28 kDa integral membrane protein on the cis-Golgi. J Cell Sci. 1995;108:2405–2414. doi: 10.1242/jcs.108.6.2405. [DOI] [PubMed] [Google Scholar]
- Subramaniam VN, Peter F, Philip R, Wong SH, Hong W. GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science. 1996;272:1161–1163. doi: 10.1126/science.272.5265.1161. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Tam SK, Gu W, Mahdavi V, Nadal-Ginard B. Cardiac myocyte terminal differentiation. Potential for cardiac regeneration. Ann NY Acad Sci. 1995;752:72–79. doi: 10.1111/j.1749-6632.1995.tb17407.x. [DOI] [PubMed] [Google Scholar]
- Tang BL, Low DY, Hong W. Hsec22c: a homolog of yeast Sec22p and mammalian rsec22a and msec22b/ERS-24. Biochem Biophys Res Commun. 1998a;243:885–891. doi: 10.1006/bbrc.1998.8194. [DOI] [PubMed] [Google Scholar]
- Tang BL, Low DY, Lee SS, Tan AE, Hong W. Molecular cloning and localization of human syntaxin 16, a member of the syntaxin family of SNARE proteins. Biochem Biophys Res Commun. 1998b;242:673–679. doi: 10.1006/bbrc.1997.8029. [DOI] [PubMed] [Google Scholar]
- Tang BL, Low DY, Tan AE, Hong W. Syntaxin 10: a member of the syntaxin family localized to the trans-Golgi network. Biochem Biophys Res Commun. 1998c;242:345–350. doi: 10.1006/bbrc.1997.7966. [DOI] [PubMed] [Google Scholar]
- Tang BL, Tan AEH, Lim LK, Lee SS, Low DYH, Hong W. Syntaxin 12, a member of the syntaxin family localized to the endosome. J Biol Chem. 1998d;273:6944–6950. doi: 10.1074/jbc.273.12.6944. [DOI] [PubMed] [Google Scholar]
- Walsh K, Perlman H. Cell cycle exit upon myogenic differentiation. Curr Opin Genet Dev. 1997;7:597–602. doi: 10.1016/s0959-437x(97)80005-6. [DOI] [PubMed] [Google Scholar]
- Wang H, Frelin L, Pevsner J. Human syntaxin 7: a Pep12p/Vps6p homologue implicated in vesicle trafficking to lysosomes. Gene. 1997;199:39–48. doi: 10.1016/s0378-1119(97)00343-0. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Whiteheart SW, Kubalek EW. SNAPs and NSF: general members of the fusion apparatus. Trends Cell Biol. 1995;5:64–69. doi: 10.1016/s0962-8924(00)88948-5. [DOI] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Hong W. Syntaxin 7, a novel syntaxin member associated with the early endosomal compartment. J Biol Chem. 1998;273:375–380. doi: 10.1074/jbc.273.1.375. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Yang W, Storrie B. Scattered Golgi elements during microtubule disruption are initially enriched in trans-Golgi proteins. Mol Biol Cell. 1998;9:191–207. doi: 10.1091/mbc.9.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotov WV, St-Arnaud R. Differential splicing-in of a proline-rich exon converts alphaNAC into a muscle-specific transcription factor. Genes Dev. 1996;10:1763–1772. doi: 10.1101/gad.10.14.1763. [DOI] [PubMed] [Google Scholar]
- Zeng Q, Carter DA, Murphy D. Cell specific expression of a vasopressin transgene in rats. J Neuroendocrinol. 1994;6:469–477. doi: 10.1111/j.1365-2826.1994.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wong SH, Tang BL, Xu Y, Peter F, Subramaniam VN, Hong W. The mammalian protein (rbet1) homologous to yeast Bet1p is primarily associated with the pre-Golgi intermediate compartment and is involved in vesicular transport from the endoplasmic reticulum to the Golgi apparatus. J Cell Biol. 1997;139:1157–1168. doi: 10.1083/jcb.139.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]