Abstract
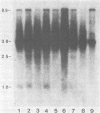
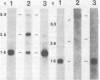
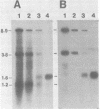
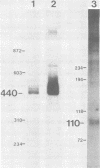
We have examined an assortment of preneoplastic and neoplastic mouse mammary tissues for the presence of an mRNA which could encode the putative long terminal repeat gene product of mouse mammary tumor virus. We report here the detection of a novel mouse mammary tumor virus-specific, polyadenylic acid-containing transcript in certain preneoplastic and neoplastic mammary tissue of BALB/c mice. The molecule is 1.6 kilobases in length and contains sequences from the transcriptional leader and the U3 region of the proviral DNA. The upstream terminus of the 3' information lies 75 to 80 nucleotides from the beginning of the long terminal repeat open reading frame, in close proximity to a consensus splice acceptor in the DNA. The transcript was detected in hormonally or chemically induced neoplastic, preneoplastic, and lactating mammary tissue of BALB/c mice, but not in preneoplastic or tumor tissue induced by exogenous viruses in any strain of mice examined. This implies that the RNA we observed is transcribed from an endogenous provirus template.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Naso R. B., Davis J., Bowen J. M. Polyprotein precursors to mouse mammary tumor virus proteins. J Virol. 1979 Nov;32(2):507–516. doi: 10.1128/jvi.32.2.507-516.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley R. L., Cardiff R. D., Mitchell D. J., Faulkin L. J., Lund J. K. Development and characterization of mouse hyperplastic mammary outgrowth lines from BALB/cfC3H hyperplastic alveolar nodules. Cancer Res. 1980 Nov;40(11):4232–4242. [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznik T., Cohen J. C. Altered methylation of endogenous viral promoter sequences during mammary carcinogenesis. Nature. 1982 Jan 21;295(5846):255–257. doi: 10.1038/295255a0. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Dusing-Swartz S., Socher S. H., Medina D. Partial expression of endogenous mouse mammary tumor virus in mammary tumors induced in BALB/c mice by chemical, hormonal, and physical agents. J Virol. 1981 May;38(2):571–580. doi: 10.1128/jvi.38.2.571-580.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Structure and processing of the mouse mammary tumor virus glycoprotein precursor pr73env. J Virol. 1980 Aug;35(2):349–361. doi: 10.1128/jvi.35.2.349-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Andre J., Berard D. S., Wolford R. G., Hager G. L. Construction and characterization of molecular clones containing integrated mouse mammary tumor virus sequences. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1153–1159. doi: 10.1101/sqb.1980.044.01.124. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Fleurdelys B., Hager G. L. Further evidence for the protein coding potential of the mouse mammary tumor virus long terminal repeat: nucleotide sequence of an endogenous proviral long terminal repeat. J Virol. 1983 Mar;45(3):941–949. doi: 10.1128/jvi.45.3.941-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drohan W., Teramoto Y. A., Medina D., Schlom J. Isolation and characterization of a new mouse mammary tumor virus from BALB/c mice. Virology. 1981 Oct 15;114(1):175–186. doi: 10.1016/0042-6822(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Dudley J. P., Varmus H. E. Purification and translation of murine mammary tumor virus mRNA's. J Virol. 1981 Jul;39(1):207–218. doi: 10.1128/jvi.39.1.207-218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusing-Swartz S., Medina D., Butel J. S., Socher S. H. Mouse mammary tumor virus genome expression in chemical carcinogen-induced mammary tumors in low- and high-tumor-incidence mouse strains. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5360–5364. doi: 10.1073/pnas.76.10.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Furth M. E., Scolnick E. M. Mouse cells contain two distinct ras gene mRNA species that can be translated into a p21 onc protein. Mol Cell Biol. 1982 Nov;2(11):1339–1345. doi: 10.1128/mcb.2.11.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. N., Pheiffer B. H., Boehnert J. A. Chemical and electrophoretic properties of solubilizable disulfide gels. Anal Biochem. 1980 Jun;105(1):192–201. doi: 10.1016/0003-2697(80)90445-5. [DOI] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Further characterization of intracellular precursor polyproteins of Rauscher leukemia virus. Virology. 1977 May 1;78(1):11–34. doi: 10.1016/0042-6822(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGrath C. M., Jones R. F. Hormonal induction of mammary tumor viruses and its implications for carcinogenesis. Cancer Res. 1978 Nov;38(11 Pt 2):4112–4125. [PubMed] [Google Scholar]
- McGrath C. M., Marineau E. J., Voyles B. A. Changes in MuMTV DNA and RNA levels in Balb/c mammary epithelial cells during malignant transformation by exogenous MuTV and by hormones. Virology. 1978 Jun 15;87(2):339–353. doi: 10.1016/0042-6822(78)90139-3. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina D., DeOme K. B. Influence of mammary tumor virus on the tumor-producing capabilities of nodule outgrowth free of mammary tumor virus. J Natl Cancer Inst. 1968 Jun;40(6):1303–1308. [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nusse R., Hageman P. Induction of mouse mammary tumor virus RNA in mammary tumors of BALB/c mice treated with urethane, X-irradiation, and hormones. J Virol. 1979 Jul;31(1):63–72. doi: 10.1128/jvi.31.1.63-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauley R. J., Medina D., Socher S. H. Murine mammary tumor virus expression during mammary tumorigenesis in BALB/c mice. J Virol. 1979 Feb;29(2):483–493. doi: 10.1128/jvi.29.2.483-493.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Dexamethasone induction of the intracellular RNAs of mouse mammary tumor virus. J Virol. 1981 Dec;40(3):673–682. doi: 10.1128/jvi.40.3.673-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Schlom J., Michalides R., Kufe D., Hehlmann R., Spiegelman S., Bentvelzen P., Hageman P. A comparative study of the biologic and molecular basis of murine mammary carcinoma: a model for human breast cancer. J Natl Cancer Inst. 1973 Aug;51(2):541–551. [PubMed] [Google Scholar]
- Sen G. C., Racevskis J., Sarkar N. H. Synthesis of murine mammary tumor viral proteins in vitro. J Virol. 1981 Mar;37(3):963–975. doi: 10.1128/jvi.37.3.963-975.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]