Abstract
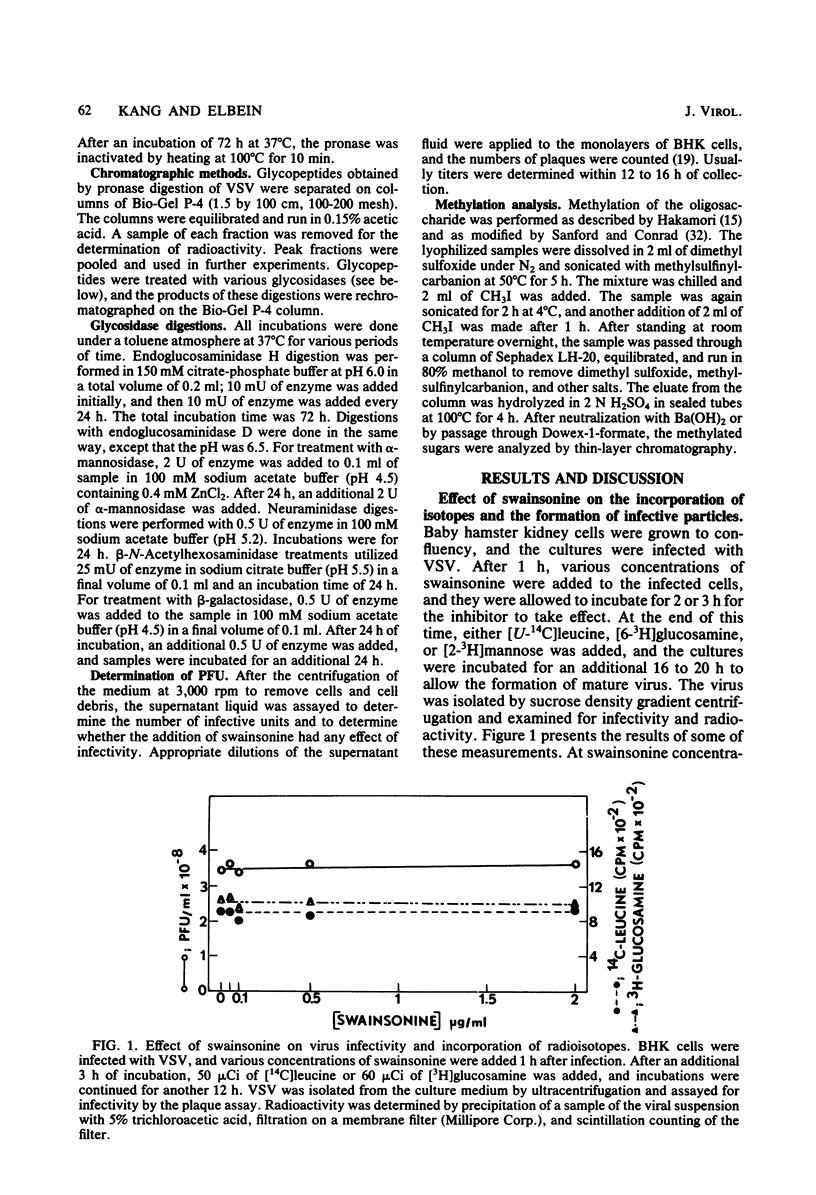
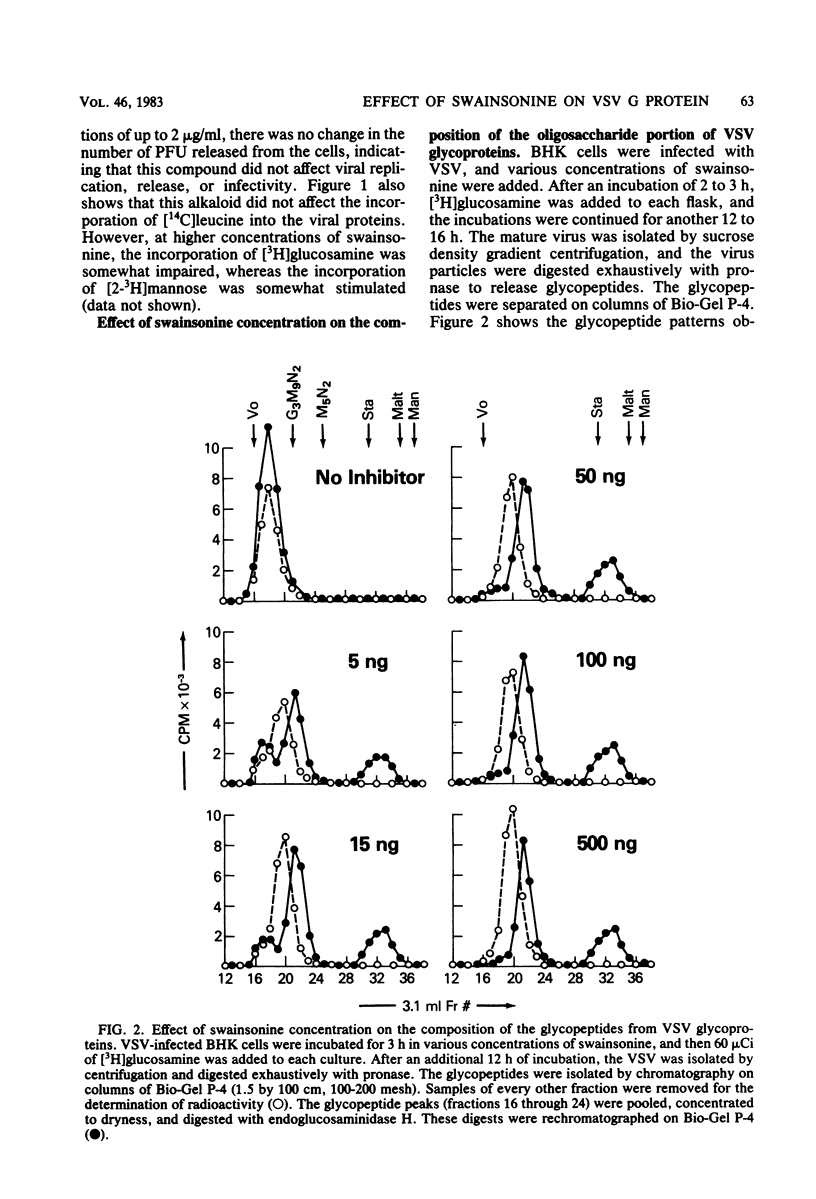
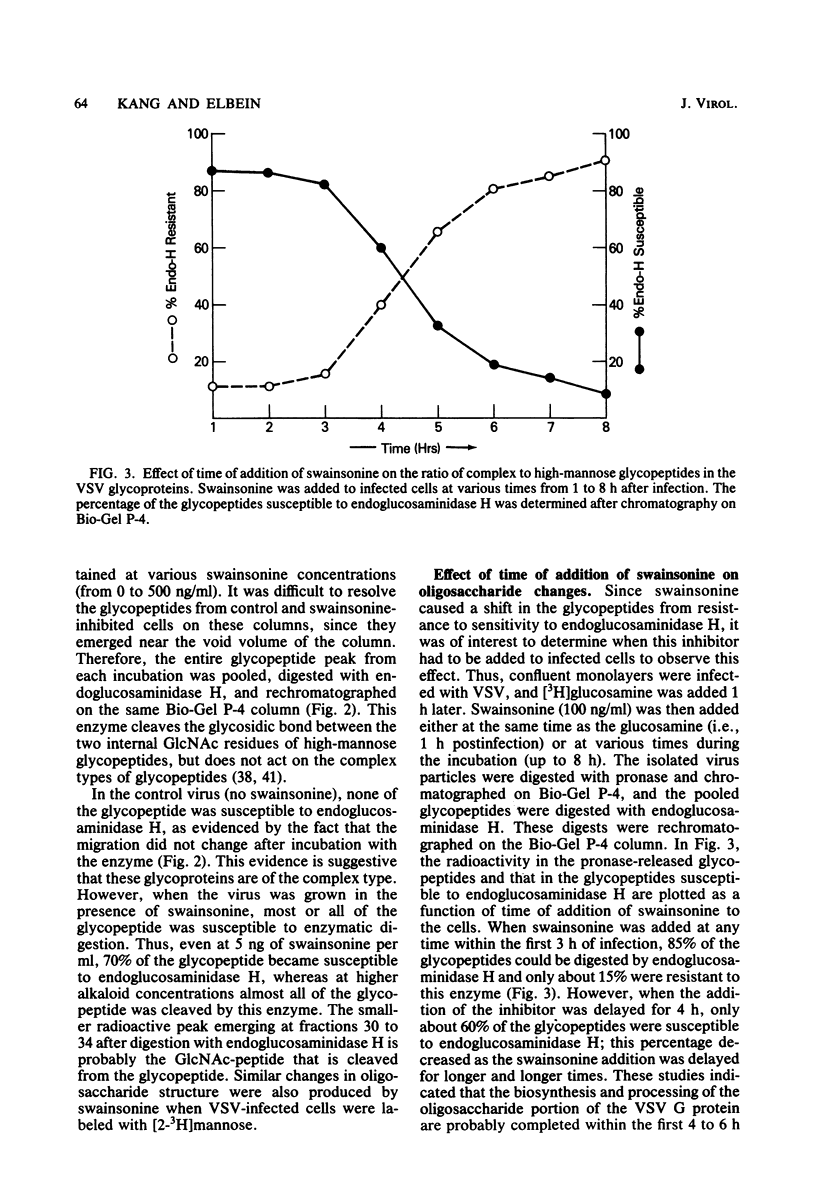
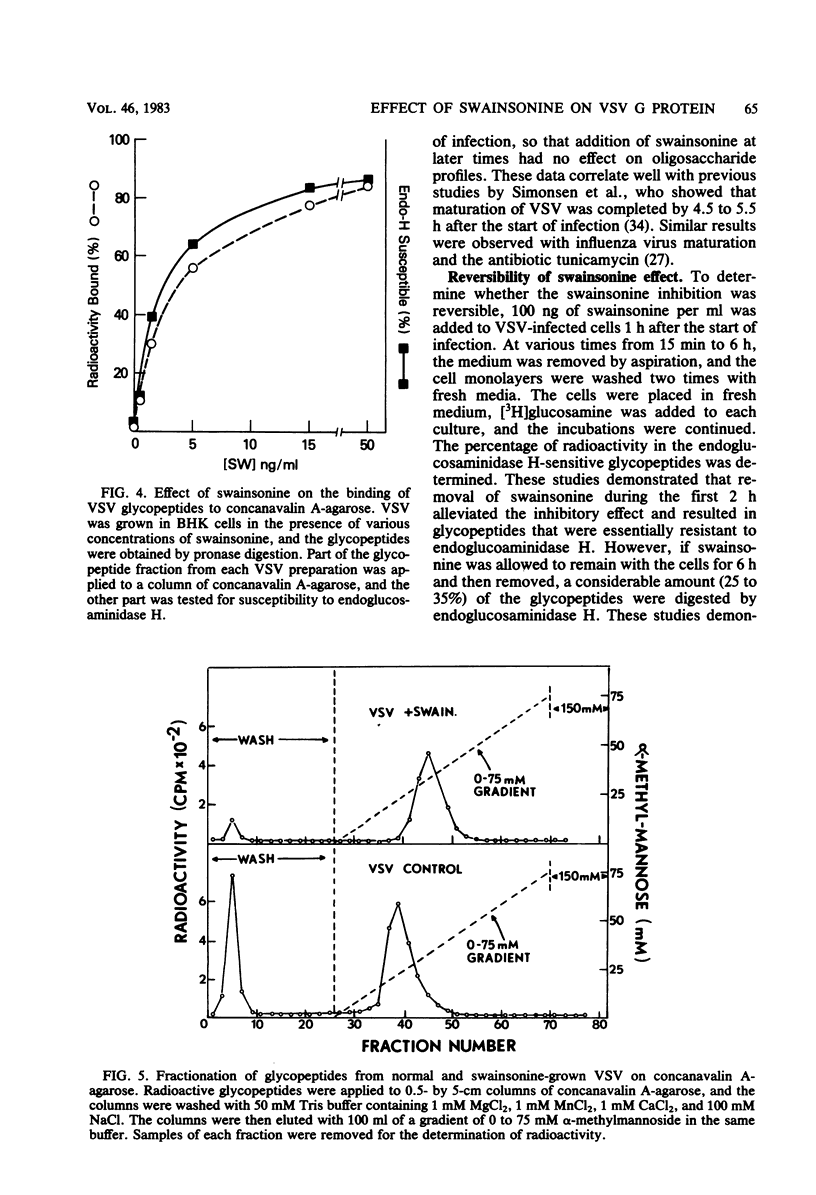
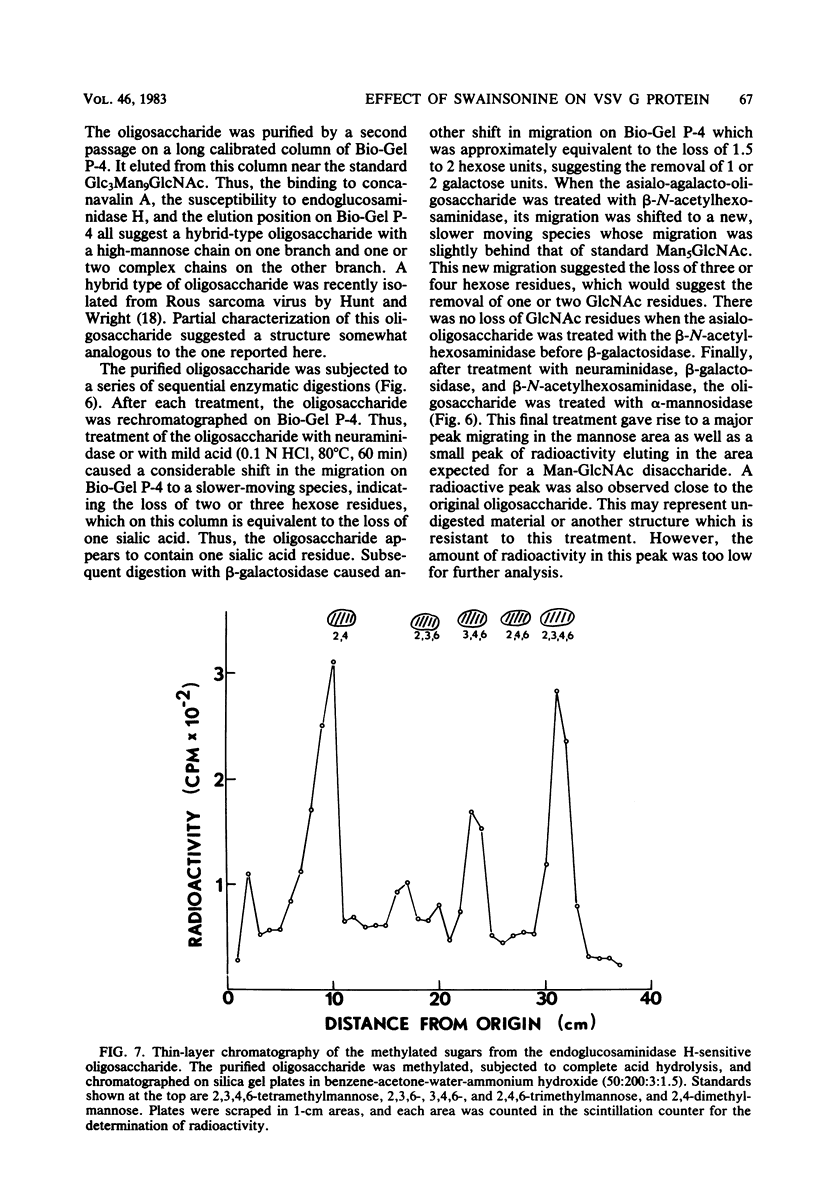
Swainsonine, an inhibitor of glycoprotein processing, inhibits the formation of the normal oligosaccharide chain of the G protein of vesicular stomatitis virus. Thus, when vesicular stomatitis virus was grown in baby hamster kidney cells in the presence of swainsonine (15 to 500 ng/ml) and labeled with [2-3H]mannose, the oligosaccharide portion of the G protein was completely susceptible to the action of endoglucosaminidase H. However, the normal viral glycoprotein is not susceptible to this enzyme. Various enzymatic treatments and methylation studies of the mannose-labeled oligosaccharides suggest that swainsonine causes the formation of a hybrid-type oligosaccharide having an oligomannosyl core (Man5GlcNAc2-Asn) characteristic of neutral oligosaccharides plus the branch structure (NeuNAc-Gal-GlcNAc) characteristic of the complex oligosaccharides. A structure for this hybrid oligosaccharide is proposed. Swainsonine had no effect on the incorporation of [14C]leucine into viral proteins, nor did it change the number of PFU produced in these cultures. It did, however, slightly decrease the incorporation of [3H]glucosamine and increase the incorporation of [3H]mannose. Vesicular stomatitis virus raised in the presence of swainsonine bound much more tightly to columns of concanavalin A-Sepharose than did control virus. Swainsonine had to be added within the first 4 or 5 h of virus infection to be effective. Thus, when 100 ng of the alkaloid per ml was added at any time within the first 3 h of infection, essentially all of the glycoprotein was susceptible to digestion by endoglucosaminidase H. However, when swainsonine was added 4 h after the start of infection, 30% of the glycopeptides became resistant to endoglucosaminidase H; at 5 h, 70% were resistant. The effect of swainsonine was reversible since removal of the alkaloid allowed the cells to form the normal complex glycoproteins. However, the time of removal was critical in terms of oligosaccharide structure.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Czichi U., Lennarz W. J. Localization of the enzyme system for glycosylation of proteins via the lipid-linked pathway in rough endoplasmic reticulum. J Biol Chem. 1977 Nov 25;252(22):7901–7904. [PubMed] [Google Scholar]
- Das R. C., Heath E. C. Dolichyldiphosphoryloligosaccharide--protein oligosaccharyltransferase; solubilization, purification, and properties. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3811–3815. doi: 10.1073/pnas.77.7.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorling P. R., Huxtable C. R., Colegate S. M. Inhibition of lysosomal alpha-mannosidase by swainsonine, an indolizidine alkaloid isolated from Swainsona canescens. Biochem J. 1980 Nov 1;191(2):649–651. doi: 10.1042/bj1910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D., Dorling P. R., Vosbeck K., Horisberger M. Swainsonine prevents the processing of the oligosaccharide chains of influenza virus hemagglutinin. J Biol Chem. 1982 Feb 25;257(4):1573–1576. [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elting J. J., Chen W. W., Lennarz W. J. Characterization of a glucosidase involved in an initial step in the processing of oligosaccharide chains. J Biol Chem. 1980 Mar 25;255(6):2325–2331. [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Summers D. F., Georgopoulos C. Variations in the structure of radiolabeled glycopeptides from the glycoprotein of vesicular stomatitis virus grown in four mouse teratocarcinoma cell lines. J Biol Chem. 1981 Apr 10;256(7):3366–3369. [PubMed] [Google Scholar]
- Forsee W. T., Schutzbach J. S. Purification and characterization of a phospholipid-dependent alpha-mannosidase from rabbit liver. J Biol Chem. 1981 Jul 10;256(13):6577–6582. [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Grinna L. S., Robbins P. W. Substrate specificities of rat liver microsomal glucosidases which process glycoproteins. J Biol Chem. 1980 Mar 25;255(6):2255–2258. [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hunt L. A., Etchison J. R., Summers D. F. Oligosaccharide chains are trimmed during synthesis of the envelope glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1978 Feb;75(2):754–758. doi: 10.1073/pnas.75.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Wright S. E. Rous sarcoma virus glycoproteins contain hybrid-type oligosaccharides. J Virol. 1981 Aug;39(2):646–650. doi: 10.1128/jvi.39.2.646-650.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M. S., Park J. J., Singh I., Phillips L. A. Streptovirudin inhibits glycosylation and multiplication of vesicular stomatitis virus. Biochem Biophys Res Commun. 1981 Mar 31;99(2):422–428. doi: 10.1016/0006-291x(81)91762-9. [DOI] [PubMed] [Google Scholar]
- Kiely M. L., McKnight G. S., Schimke R. T. Studies on the attachment of carbohydrate to ovalbumin nascent chains in hen oviduct. J Biol Chem. 1976 Sep 25;251(18):5490–5495. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Weiss R. A. Selective isolation of mutants of vesicular stomatitis virus defective in production of the viral glycoprotein. J Virol. 1979 Apr;30(1):177–189. doi: 10.1128/jvi.30.1.177-189.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S., Stanley P., Schachter H. Control of glycoprotein synthesis. Lectin-resistant mutant containing only one of two distinct N-acetylglucosaminyltransferase activities present in wild type Chinese hamster ovary cells. J Biol Chem. 1977 Jun 10;252(11):3926–3933. [PubMed] [Google Scholar]
- Opheim D. J., Touster O. Lysosomal alpha-D-mannosidase of rat liver. Purification and comparison with the golgi and cytosolic alpha-D-mannosidases. J Biol Chem. 1978 Feb 25;253(4):1017–1023. [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Robertson M. A., Etchison J. R., Robertson J. S., Summers D. F., Stanley P. Specific changes in the oligosaccharide moieties of VSV grown in different lectin-resistnat CHO cells. Cell. 1978 Mar;13(3):515–526. doi: 10.1016/0092-8674(78)90325-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Kreibich G., Sabatini D. D. Spatial orientation of glycoproteins in membranes of rat liver rough microsomes. I. Localization of lectin-binding sites in microsomal membranes. J Cell Biol. 1978 Sep;78(3):874–893. doi: 10.1083/jcb.78.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Hill V. M., Summers D. F. Further characterization of the replicative complex of vesicular stomatitis virus. J Virol. 1979 Aug;31(2):494–505. doi: 10.1128/jvi.31.2.494-505.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Ugalde R. A., Staneloni R. J., Leloir L. F. Action of glycosidases on the saccharide moiety of the glucose--containing dolichyl diphosphate oligosaccharide. FEBS Lett. 1978 Jul 15;91(2):209–212. doi: 10.1016/0014-5793(78)81174-0. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Kobata A. The structures of the galactose-containing sugar chains of ovalbumin. J Biol Chem. 1978 Jun 10;253(11):3862–3869. [PubMed] [Google Scholar]


