Abstract
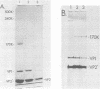
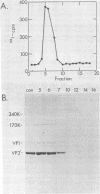
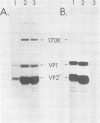
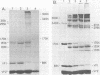
The structure of the icosahedral capsid of the H-1 parvovirus was probed by chemical cross-linking methods. Treatment of empty capsids with high-molecular-weight polyethylene glycols resulted in irreversible aggregation of the minor capsid protein VP1. Multimers of VP1 containing at least five and perhaps six molecules were obtained, but only with empty capsids and not with the full, DNA-containing virus. Cross-linking of the empty capsids with dimethylsuberimidate confirmed the assignments of the products formed after treatment with polyethylene glycol. With dimethylsuberimidate the most abundant product was a heterologous dimer containing VP1 and the major capsid protein VP2'. A small amount of homologous VP2' dimer was also obtained, but the majority of VP2' remained unreacted even at high concentrations of dimethylsuberimidate. The capsid proteins of the full virus, on the other hand, were completely unreactive to dimethylsuberimidate. The data suggest that the minor protein VP1 may be clustered in the capsid and perhaps composes one or two of the morphological units of the icosahedral shell.
Full text
PDF

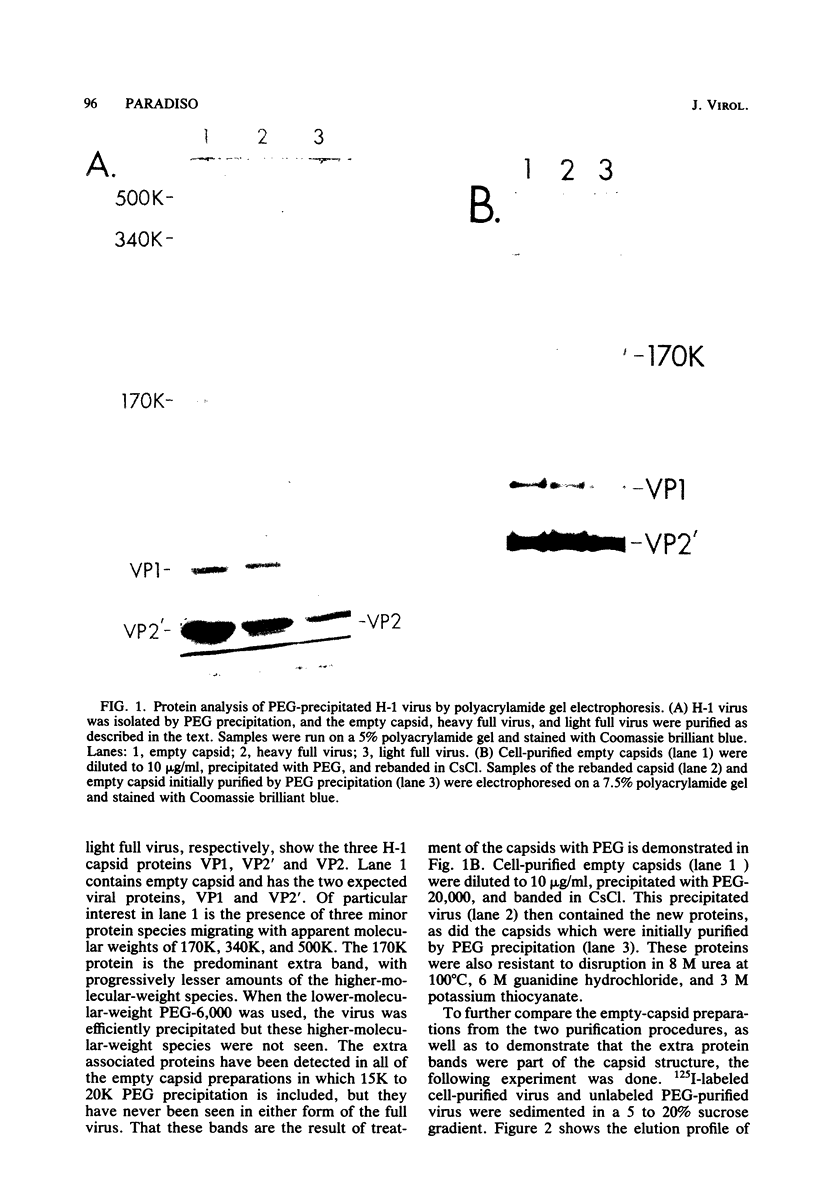
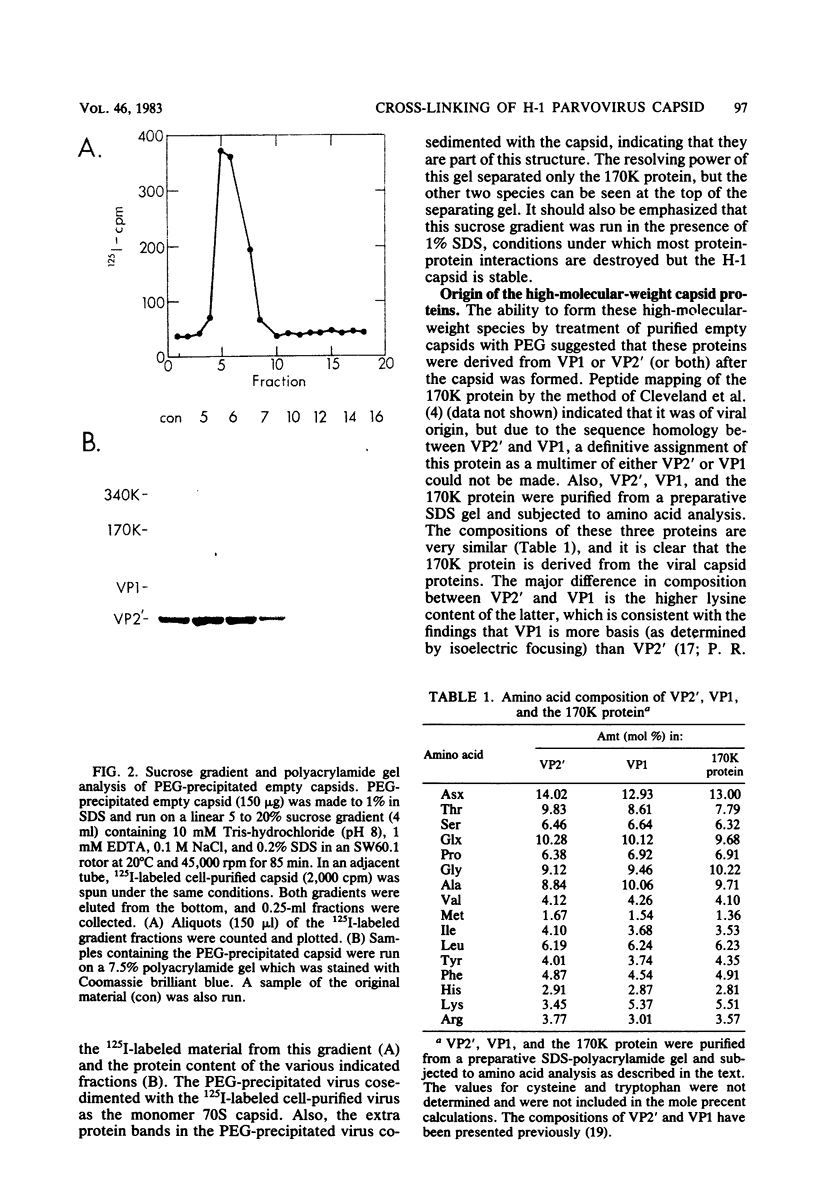

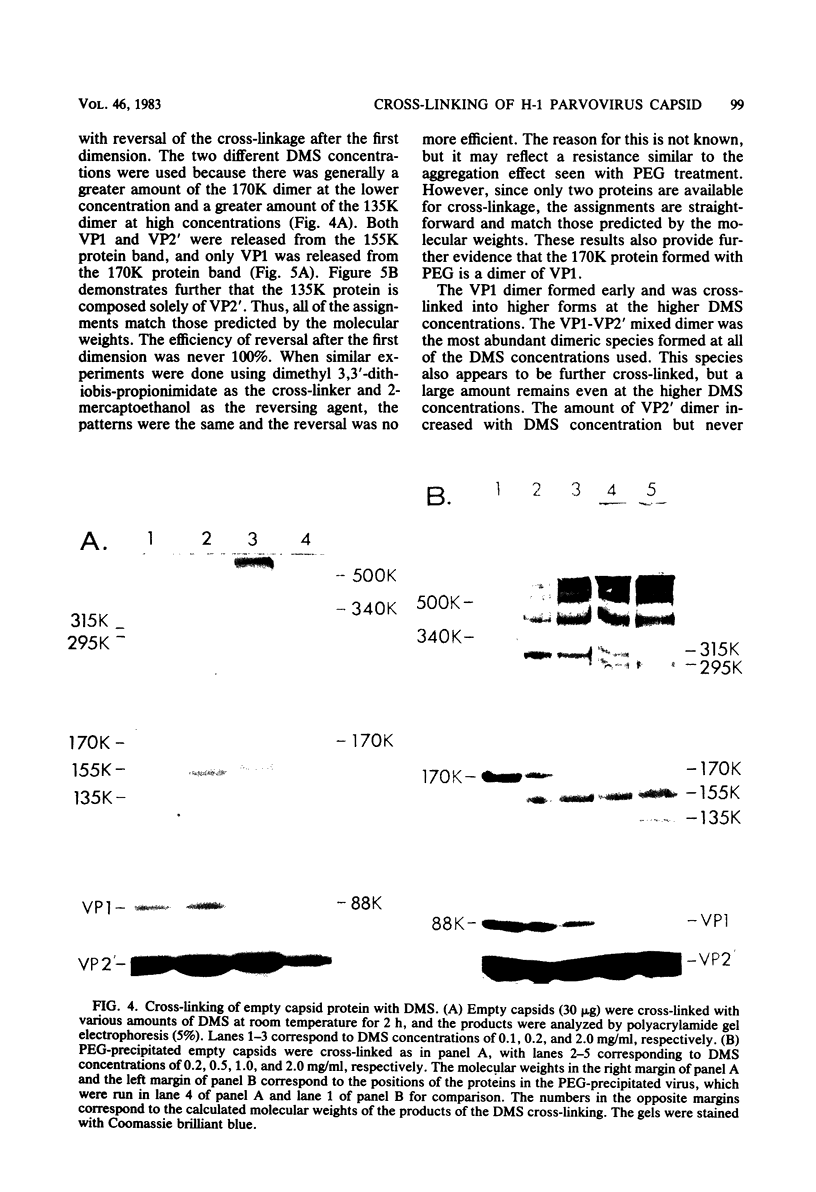



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atha D. H., Ingham K. C. Mechanism of precipitation of proteins by polyethylene glycols. Analysis in terms of excluded volume. J Biol Chem. 1981 Dec 10;256(23):12108–12117. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinton G. M., Hayashi M. The parovivirus MVM: particles with altered structural proteins. Virology. 1975 Jul;66(1):261–261. doi: 10.1016/0042-6822(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Dunker A. K. The structure of picornaviruses: classification of the bonding networks. Virology. 1979 Aug;97(1):141–150. doi: 10.1016/0042-6822(79)90380-5. [DOI] [PubMed] [Google Scholar]
- Gibson D. R., Gracy R. W. Extraction of proteins and peptides from Coomassie Blue-stained sodium dodecyl sulfate--polyacrylamide gels. Anal Biochem. 1979 Jul 15;96(2):352–354. doi: 10.1016/0003-2697(79)90592-x. [DOI] [PubMed] [Google Scholar]
- Hamburger R., Azaz E., Donbrow M. Autoxidation of polyoxyethylenic non-ionic surfactants and of polyethylene glycols. Pharm Acta Helv. 1975;50(1-2):10–17. [PubMed] [Google Scholar]
- Honda K., Maeda Y., Sasakawa S., Ohno H., Tsuchida E. The components contained in polyethylene glycol of commercial grade (PEG-6,000) as cell fusogen. Biochem Biophys Res Commun. 1981 Jul 16;101(1):165–171. doi: 10.1016/s0006-291x(81)80025-3. [DOI] [PubMed] [Google Scholar]
- Hordern J. S., Leonard J. D., Scraba D. G. Structure of the mengo virion. VI. Spatial relationships of the capsid polypeptides as determined by chemical cross-linking analyses. Virology. 1979 Aug;97(1):131–140. doi: 10.1016/0042-6822(79)90379-9. [DOI] [PubMed] [Google Scholar]
- Karasaki S. Size and ultrastructure of the H-viruses as determined with the use of specific antibodies. J Ultrastruct Res. 1966 Sep;16(1):109–122. doi: 10.1016/s0022-5320(66)80026-6. [DOI] [PubMed] [Google Scholar]
- Kongsvik J. R., Gierthy J. F., Rhode S. L., 3rd Replication process of the parvovirus H-1. IV. H-1-specific proteins synthesized in synchronized human NB kidney cells. J Virol. 1974 Dec;14(6):1600–1603. doi: 10.1128/jvi.14.6.1600-1603.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mayor H. D., Jamison R. M., Jordan L. E., Melnick J. L. Structure and Composition of a Small Particle Prepared from a Simian Adenovirus. J Bacteriol. 1965 Jul;90(1):235–242. doi: 10.1128/jb.90.1.235-242.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Infectious process of the parvovirus H-1: correlation of protein content, particle density, and viral infectivity. J Virol. 1981 Sep;39(3):800–807. doi: 10.1128/jvi.39.3.800-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. I. Kinetics in a parasynchronous cell system. J Virol. 1973 Jun;11(6):856–861. doi: 10.1128/jvi.11.6.856-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Usategui-Gomez M., Toolan H. W., Ledinko N., al-Lami F., Hopkins M. S. Single-stranded DNA from the Parvovirus, H-1. Virology. 1969 Nov;39(3):617–621. doi: 10.1016/0042-6822(69)90117-2. [DOI] [PubMed] [Google Scholar]
- VASQUEZ C., BRAILOVSKY C. PURIFICATION AND FINE STRUCTURE OF KILHAM'S RAT VIRUS. Exp Mol Pathol. 1965 Feb;76:130–140. doi: 10.1016/0014-4800(65)90029-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]