Abstract
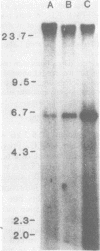
We used Southern blot hybridization to titrate and map restriction enzyme cleavage sites of a 6.3-kilobase-pair species of extrachromosomal viral DNA found in derivatives of the 745A line of murine erythroleukemia cells, which vary in their ability to be induced to differentiate by dimethyl sulfoxide (DMSO). Greater than an eightfold variation was observed in the amount of this DNA, with the largest amounts being found in cells that were resistant to the induction of differentiation by DMSO. This increase in the level of extrachromosomal viral DNA was found to be dependent upon the continued presence of DMSO in the culture medium. The increase was shown not to be due to an immediate stimulatory effect of this agent on the synthesis or maintenance of this DNA, since cell lines sensitive to the differentiation-inducing effects of DMSO were shown to undergo a transient reduction in the amount of extrachromosomal viral DNA after the addition of DMSO to the culture medium. In addition to the 6.3-kilobase-pair linear form found in the cytoplasm, in some preparations two hybridizing bands were observed that migrated in agarose gels in the position expected of covalently closed circular species of viral DNA. Restriction enzyme mapping of the cytoplasmic linear form indicated a close relationship of this DNA to two polycythemic strains of spleen focus-forming virus that have been molecularly cloned by other workers. No obvious change in the number or arrangement of chromosomal viral sequences could be detected after treating cells with DMSO. Thus, the exposure of murine erythroleukemia cells to DMSO caused an obvious change in the amount of extrachromosomal spleen focus-forming virus DNA but no obvious change in the integration of the provirus.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D. E., Terry R., Kern F. G. Cell differentiation rates of Friend murine erythroleukemia variants isolated by sib selection. Somatic Cell Genet. 1979 Sep;5(5):539–549. doi: 10.1007/BF01542693. [DOI] [PubMed] [Google Scholar]
- Canaani E., Aaronson S. A. Restriction enzyme analysis of mouse cellular type C viral DNA: emergence of new viral sequences in spontaneous AKR/J lymphomas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1677–1681. doi: 10.1073/pnas.76.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Gupta S., Rands E., Lowy D. R. Origin of mink cytopathic focus-forming (MCF) viruses:comparison with ecotropic and xenotropic murine leukemia virus genomes. Virology. 1981 Sep;113(2):465–483. doi: 10.1016/0042-6822(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Chiswell D. J., Enrietto P. J., Evans S., Quade K., Wyke J. A. Molecular mechanisms involved in morphological variation of avian sarcoma virus-infected rat cells. Virology. 1982 Jan 30;116(2):428–440. doi: 10.1016/0042-6822(82)90137-4. [DOI] [PubMed] [Google Scholar]
- Chouikh Y., Volovitch M., Yot P. A simple and fast electrophoretic method for elution of nucleic acids from gels. Mol Biol Rep. 1979 Dec 31;5(4):237–239. doi: 10.1007/BF00782896. [DOI] [PubMed] [Google Scholar]
- Colletta G., Fragomele F., Sandomenico M. L., Vecchio G. Enhanced expression of viral polypeptides and messenger RNA in dimethyl sulfoxide and bromodeoxyuridine-treated Friend erythroleukemic cells. Exp Cell Res. 1979 Mar 15;119(2):253–264. doi: 10.1016/0014-4827(79)90353-7. [DOI] [PubMed] [Google Scholar]
- Collins C. J., Boettiger D., Green T. L., Burgess M. B., Devlin H., Parsons J. T. Arrangement of integrated avian sarcoma virus DNA sequences within the cellular genomes of transformed and revertant mammalian cells. J Virol. 1980 Feb;33(2):760–768. doi: 10.1128/jvi.33.2.760-768.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. K., Pragnell I. B., Kluge N., Gaedicke G., Steinheider G., Ostertag W. Induction of endogenous and of spleen focus-forming viruses during dimethylsulfoxide-induced differentiation of mouse erythroleukemia cells transformed by spleen focus-forming virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1863–1867. doi: 10.1073/pnas.72.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E. A., Schildkraut C. L. Lengthening of the G1 phase is not strictly correlated with differentiation in Friend erythroleukemia cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3813–3817. doi: 10.1073/pnas.75.8.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Patuleia M. C., De Harven E. Erythrocytic maturation in vitro of murine (Friend) virus-induced leukemic cells. Natl Cancer Inst Monogr. 1966 Sep;22:505–522. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde D. W., Bersch N., Friend C., Tsuei D., Marovitz W. Transformation of DBA/2 mouse fetal liver cells infected in vitro by the anemic strain of Friend leukemia virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):962–966. doi: 10.1073/pnas.76.2.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Greiser-Wilke I., Ostertag W., Goldfarb P., Lang A., Furusawa M., Conscience J. F. Inducibility of spleen focus-forming virus by BrdUrd is controlled by the differentiated state of the cell. Proc Natl Acad Sci U S A. 1981 May;78(5):2995–2999. doi: 10.1073/pnas.78.5.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J., Geller R., Clarke B., Weeks V., Housman D. Commitment to erythroid differentiation by friend erythroleukemia cells: a stochastic analysis. Cell. 1976 Oct;9(2):221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Furusawa M., Sugano H. Erythrocyte membrane-specific antigens in Friend virus-induced leukemia cells. Bibl Haematol. 1973;39:955–967. doi: 10.1159/000427928. [DOI] [PubMed] [Google Scholar]
- Ikawa Y., Kobayashi Y., Obinata M., Harada F., Hino S., Yoshikura H. RNA sequences and proteins specific to Friend strain of spleen focus-forming virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):875–885. doi: 10.1101/sqb.1980.044.01.094. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Menke J. G., Scolnick E. M. Recovery of biologically active spleen focus-forming virus from molecularly cloned spleen focus-forming virus-pBR322 circular DNA by cotransfection with infectious type C retroviral DNA. J Virol. 1980 Sep;35(3):710–721. doi: 10.1128/jvi.35.3.710-721.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. E., Reynolds F. H., Jr, Van de Ven W. J., Stephenson J. R., Mak T. W., Bernstein A. Anemia- and polycythemia-inducing isolates of Friend spleen focus-forming virus. Biological and molecular evidence for two distinct viral genomes. J Exp Med. 1980 Jun 1;151(6):1477–1492. doi: 10.1084/jem.151.6.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Melderis H., Steinheider G., Kluge N., Dube S. Synthesis of mouse haemoglobin and globin mRNA in leukaemic cell cultures. Nat New Biol. 1972 Oct 25;239(95):231–234. doi: 10.1038/newbio239231a0. [DOI] [PubMed] [Google Scholar]
- Ostertag W., Pragnell I. B. Changes in genome composition of the Friend virus complex in erythroleukemia cells during the course of differentiation induced by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3278–3282. doi: 10.1073/pnas.75.7.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Rifkind R. A., Marks P. A. Chemically induced murine erythroleukemic differentiation. Biochim Biophys Acta. 1980 Sep 22;605(3):325–346. doi: 10.1016/0304-419x(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Yamamoto K. R. Production of unintegrated mouse mammary tumor virus DNA in infected rat hepatoma cells is a secondary action of dexamethasone. J Virol. 1978 Apr;26(1):93–101. doi: 10.1128/jvi.26.1.93-101.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Friend C., De Harven E. Ultrastructural changes in Friend erythroleukemia cells treated with dimethyl sulfoxide. Cancer Res. 1971 Oct;31(10):1402–1417. [PubMed] [Google Scholar]
- Sherton C. C., Evans L. H., Polonoff E., Kabat D. Relationship of Friend murine leukemia virus production to growth and hemoglobin synthesis in cultured erythroleukemia cells. J Virol. 1976 Jul;19(1):118–125. doi: 10.1128/jvi.19.1.118-125.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Terada M., Fried J., Nudel U., Rifkind R. A., Marks P. A. Transient inhibition of initiation of S-phase associated with dimethyl sulfoxide induction of murine erythroleukemia cells to erythroid differentiation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):248–252. doi: 10.1073/pnas.74.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Ruscetti S. K., Linemeyer D. L., Scolnick E. M. Helper-independent and replication-defective erythroblastosis-inducing viruses contained within anemia-inducing Friend virus complex (FV-A). Virology. 1980 Apr 15;102(1):28–45. doi: 10.1016/0042-6822(80)90067-7. [DOI] [PubMed] [Google Scholar]
- Tsuei D., Haubenstock H., Revoltella R., Friend C. Virus production and hemoglobin synthesis in variant lines of dimethyl sulfoxide-treated Friend erythroleukemia cells. J Virol. 1979 Jul;31(1):178–183. doi: 10.1128/jvi.31.1.178-183.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Padgett T., Heasley S., Simon G., Bishop J. M. Cellular functions are required for the synthesis and integration of avian sarcoma virus-specific DNA. Cell. 1977 Jun;11(2):307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- Volloch V., Housman D. Terminal differentiation of murine erythroleukemia cells: physical stabilization of end-stage cells. J Cell Biol. 1982 May;93(2):390–394. doi: 10.1083/jcb.93.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]