Abstract
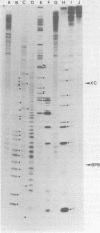
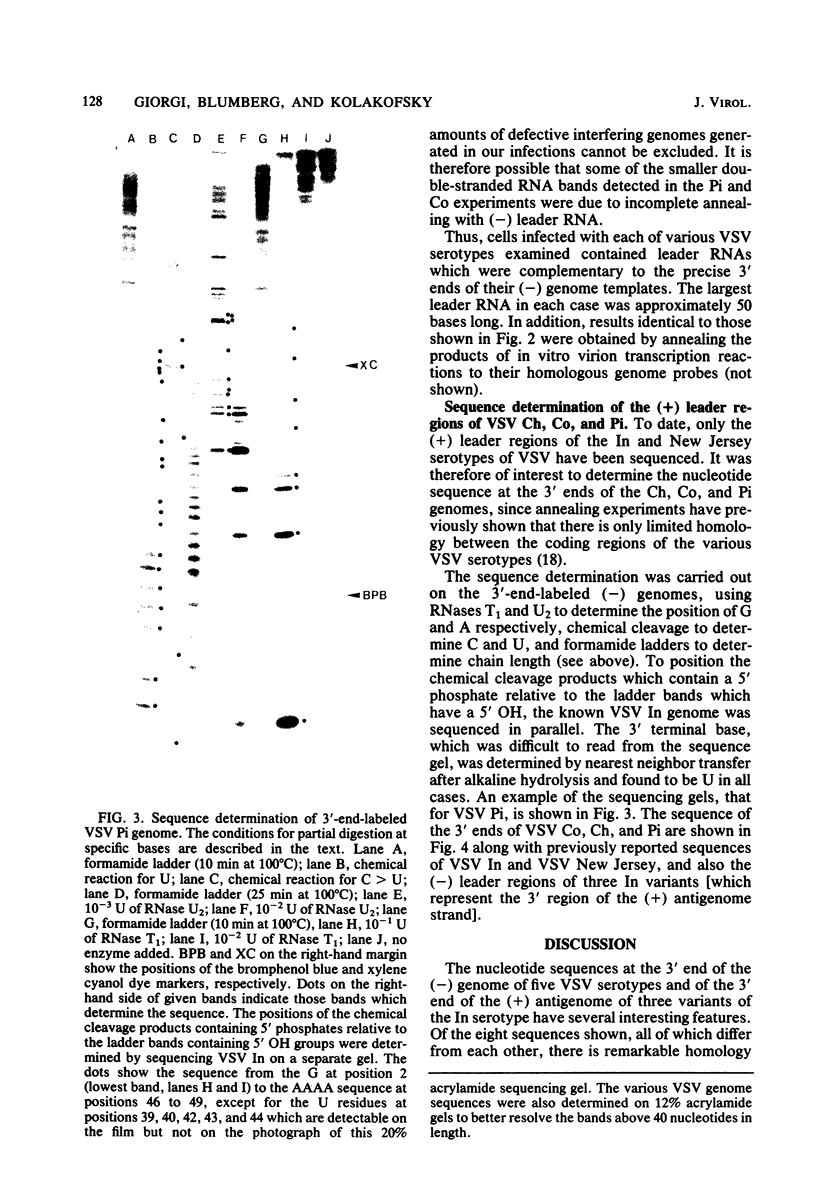
Using 3′-end-labeled genome probes, cells infected with vesicular stomatitis virus Chandipura, Cocal, and Piry serotypes were shown to contain (+) leader RNAs of approximately 50 nucleotides in length. The nucleotide sequence of the leader RNA regions of these genomes was determined and compared with the previously reported sequences of both the (+) and (-) leader RNA regions of other vesicular stomatitis virus serotypes. Regions of strong conservation of nucleotide sequence among the various vesicular stomatitis virus serotypes suggest those nucleotides thought to be involved in control functions during vesicular stomatitis virus replication.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Kolakofsky D. Intracellular vesicular stomatitis virus leader RNAs are found in nucleocapsid structures. J Virol. 1981 Nov;40(2):568–576. doi: 10.1128/jvi.40.2.568-576.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Mapping and initiation studies on the leader RNA of vesicular stomatitis virus. Virology. 1977 Mar;77(1):260–268. doi: 10.1016/0042-6822(77)90423-8. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac C. L., Keene J. D. RNA polymerase-associated interactions near template promoter sequences of defective interfering particles of vesicular stomatitis virus. J Virol. 1982 Jul;43(1):241–249. doi: 10.1128/jvi.43.1.241-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Intervening sequence between the leader region and the nucleopcapsid gene of vesicular stomatitis virus RNA. J Virol. 1980 Feb;33(2):789–794. doi: 10.1128/jvi.33.2.789-794.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Terminal sequences of vesicular stomatitis virus RNA are both complementary and conserved. J Virol. 1979 Oct;32(1):167–174. doi: 10.1128/jvi.32.1.167-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene J. D., Thornton B. J., Emerson S. U. Sequence-specific contacts between the RNA polymerase of vesicular stomatitis virus and the leader RNA gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6191–6195. doi: 10.1073/pnas.78.10.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolakofsky D. Isolation of vesicular stomatitis virus defective interfering genomes with different amounts of 5'-terminal complementarity. J Virol. 1982 Feb;41(2):566–574. doi: 10.1128/jvi.41.2.566-574.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp G., Gross H. J. Rapid RNA sequencing: nucleases from Staphylococcus aureus and Neurospora crassa discriminate between uridine and cytidine. Nucleic Acids Res. 1979 Aug 10;6(11):3481–3490. doi: 10.1093/nar/6.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leppert M., Kolakofsky D. Effect of defective interfering particles on plus- and minus- strand leader RNAs in vesicular stomatitis virus-infected cells. J Virol. 1980 Sep;35(3):704–709. doi: 10.1128/jvi.35.3.704-709.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Model P., Webster R. E., Zinder N. D. Characterization of Op3, a lysis-defective mutant of bacteriophage f2. Cell. 1979 Oct;18(2):235–246. doi: 10.1016/0092-8674(79)90043-6. [DOI] [PubMed] [Google Scholar]
- Obijeski J. F., Bishop D. H., Murphy F. A., Palmer E. L. Structural proteins of La Crosse virus. J Virol. 1976 Sep;19(3):985–997. doi: 10.1128/jvi.19.3.985-997.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repik P., Flamand A., Clark H. F., Obijeski J. F., Roy P., Bishop D. H. Detection of homologous RNA sequences among six rhabdovirus genomes. J Virol. 1974 Jan;13(1):250–252. doi: 10.1128/jvi.13.1.250-252.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Harmison G. G., Sprague J., Condra C. S., Lazzarini R. A. In vitro transcription of vesicular stomatitis virus: initiation with GTP at a specific site within the N cistron. J Virol. 1982 Jul;43(1):166–173. doi: 10.1128/jvi.43.1.166-173.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Holland J. J. Persistent vesicular stomatitis virus infection mediates base substitutions in viral RNA termini. J Virol. 1979 Nov;32(2):420–428. doi: 10.1128/jvi.32.2.420-428.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]