Abstract
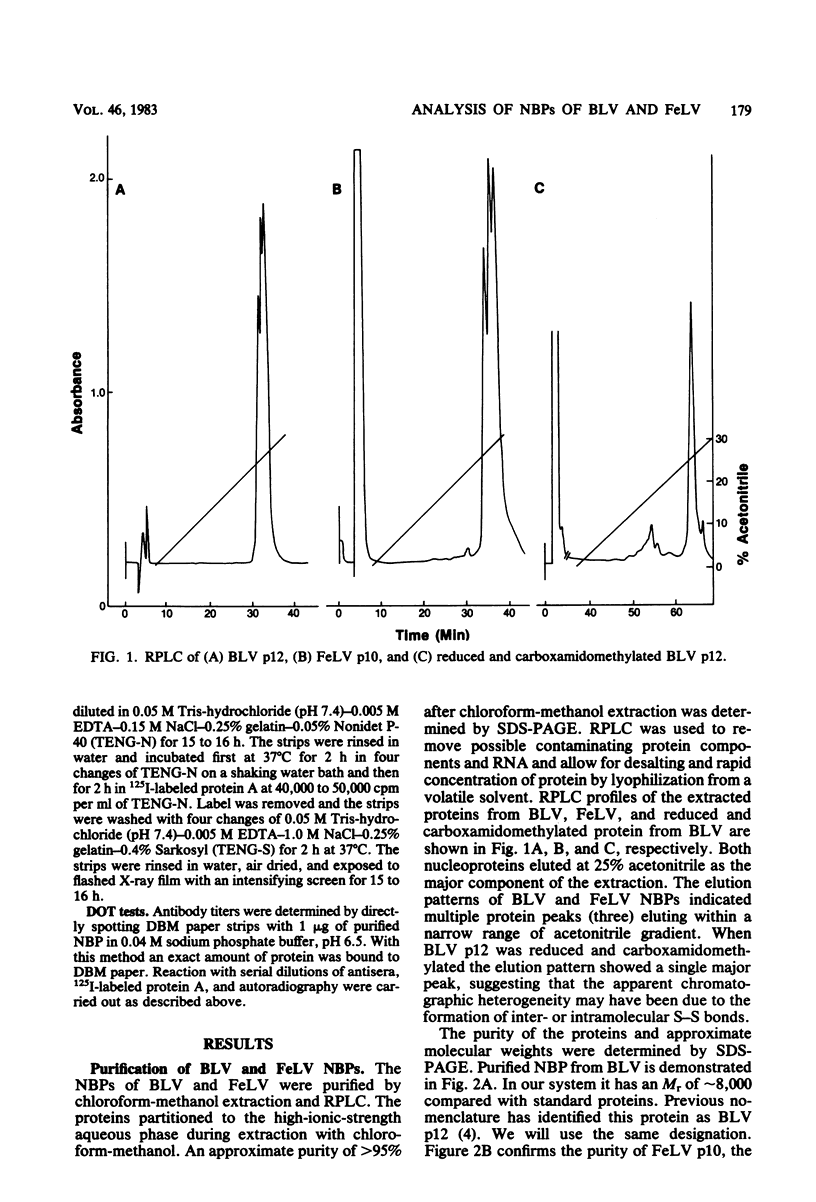
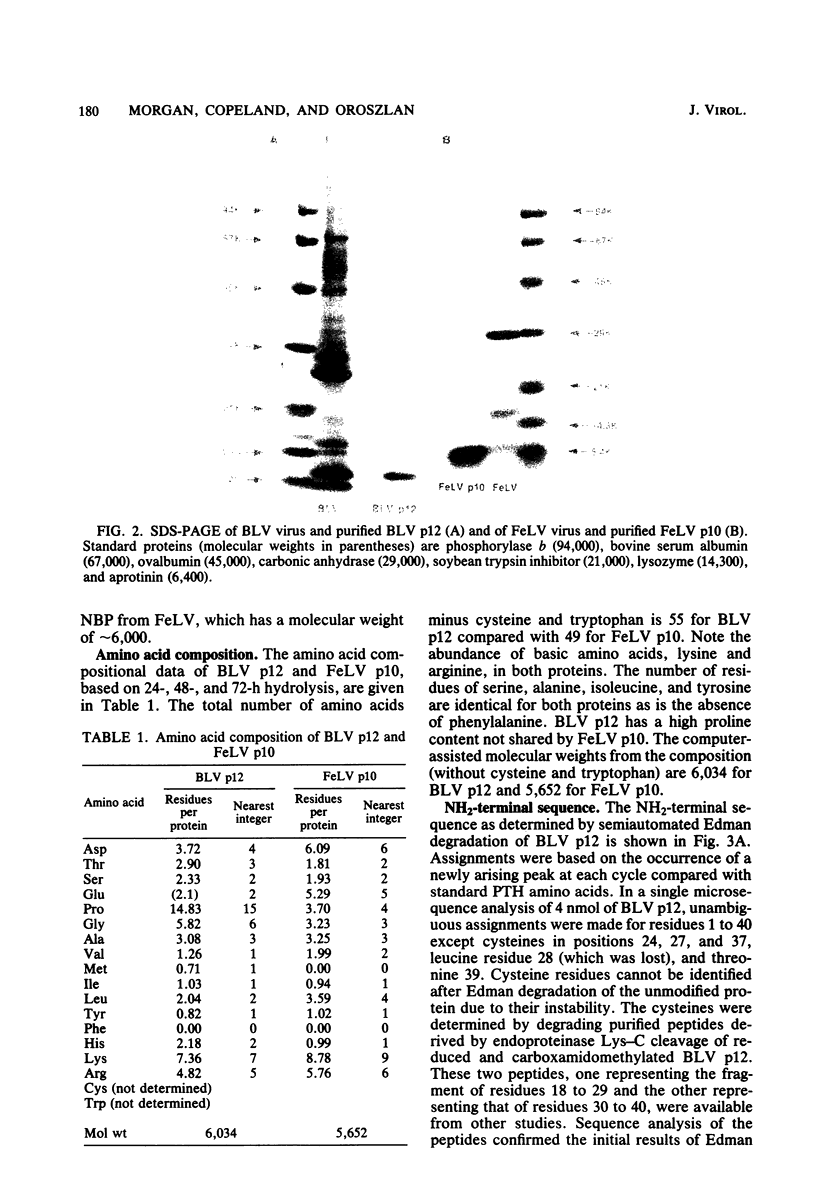
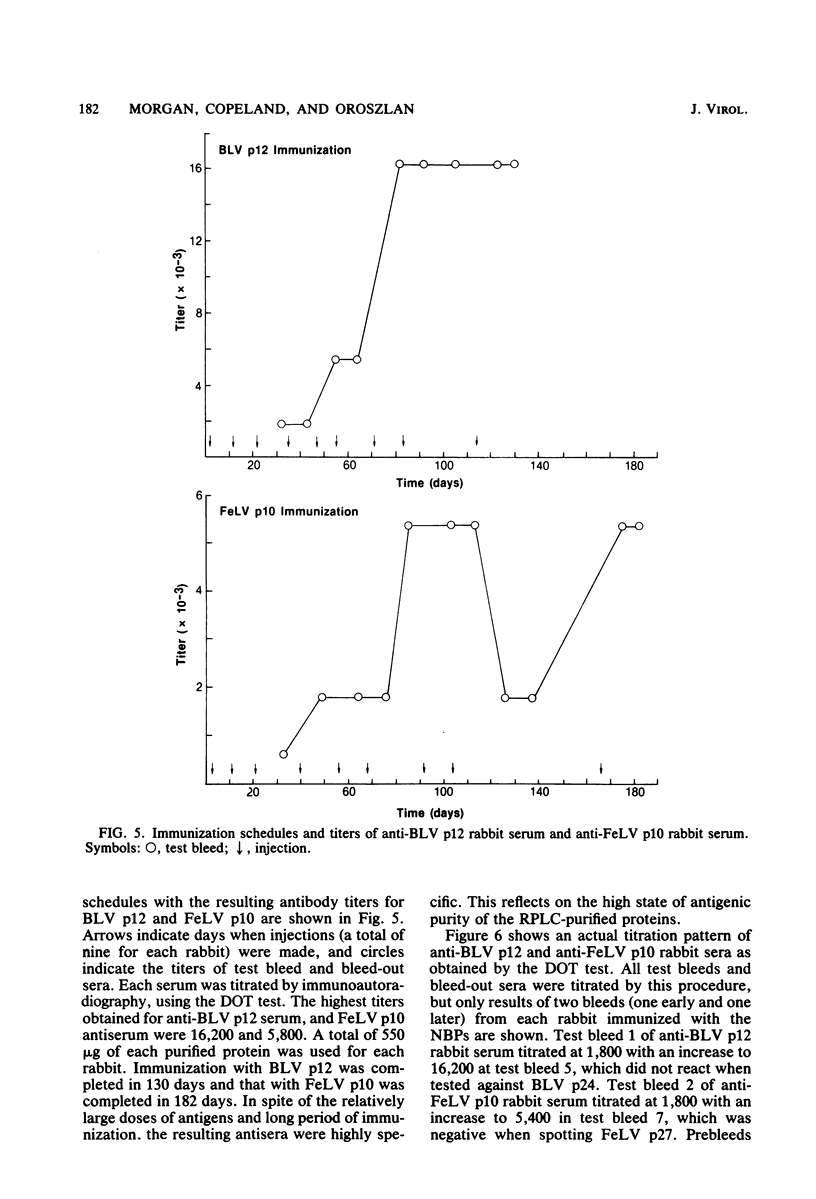
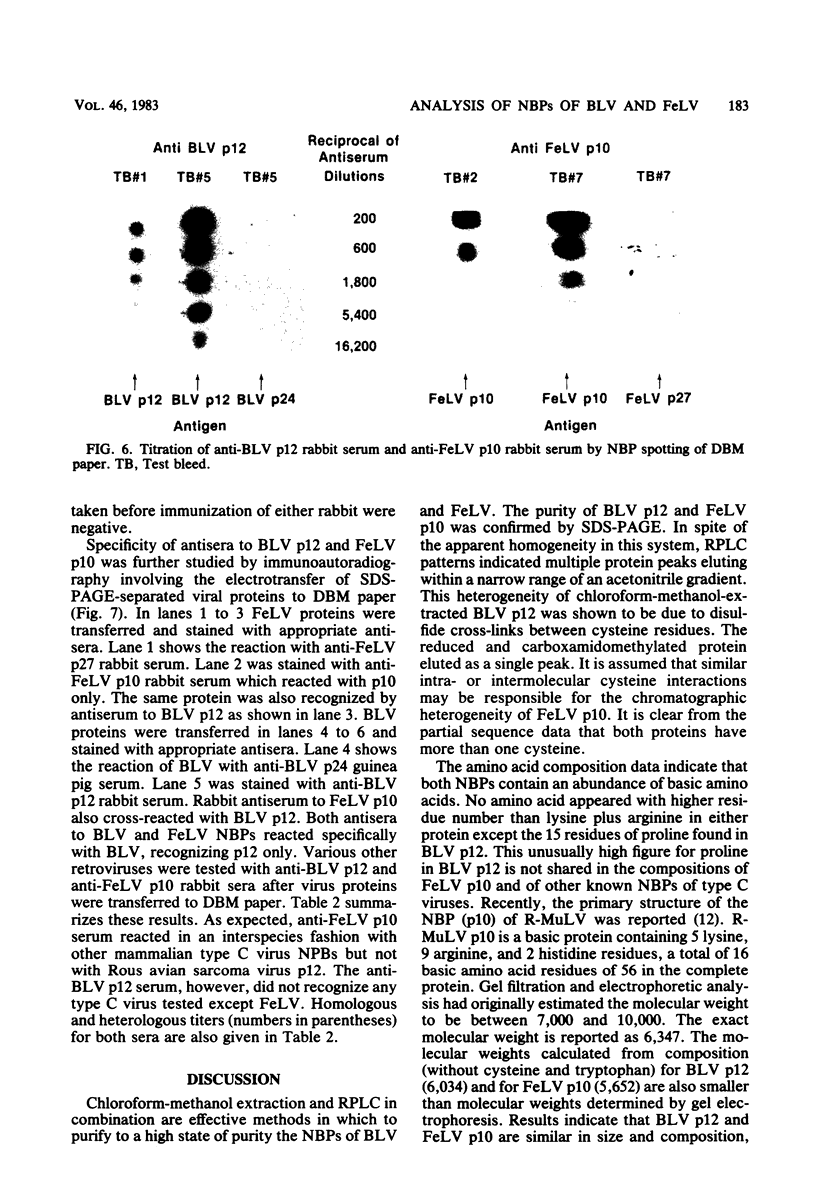
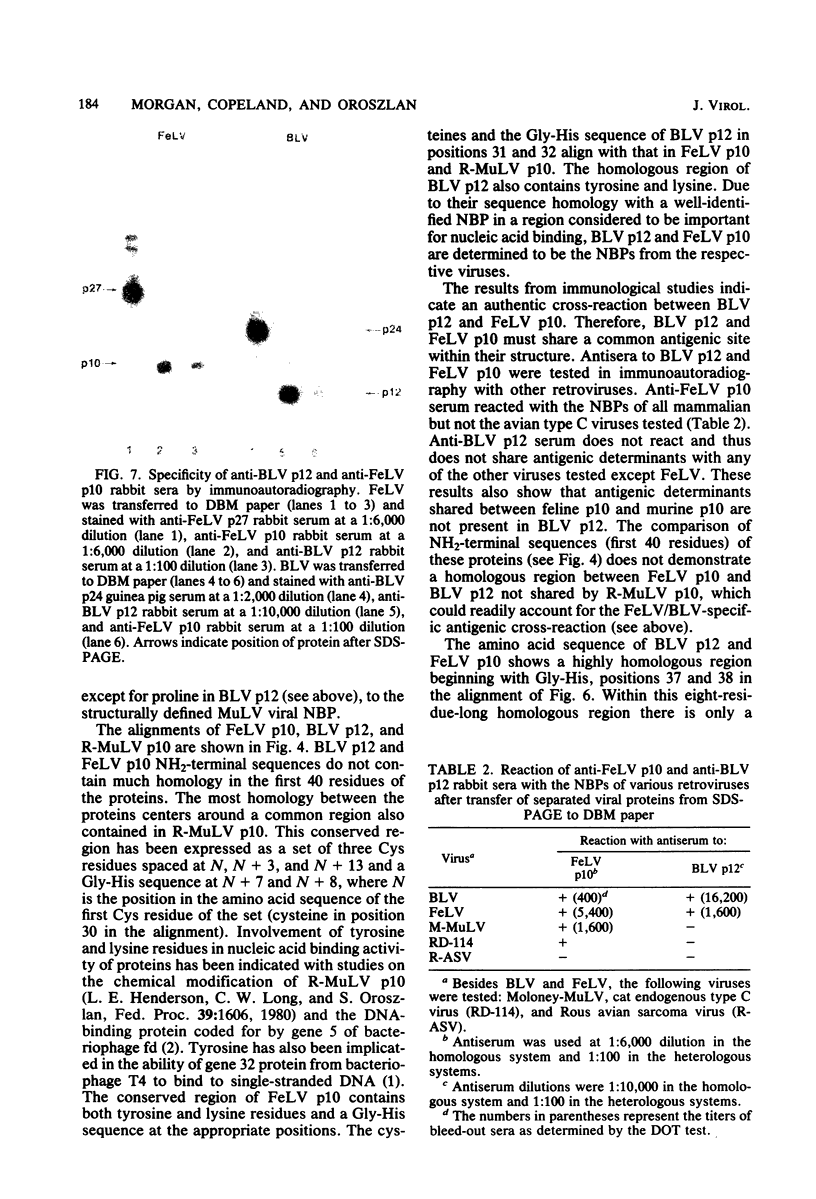
The nucleic acid-binding proteins of bovine leukemia virus (BLV) and feline leukemia virus (FeLV) were isolated in a high state of purity with chloroform-methanol extraction followed by reversed-phase liquid chromatography. Selective solubilization and purity of BLV p12 and FeLV p10 was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The compositions and molecular weights were determined by amino acid analysis. An abundance of lysine and arginine residues along with their size identifies both BLV p12 and FeLV p10 as small basic proteins similar to well-defined type C viral nucleoproteins. NH2-terminal degradation by the semiautomated Edman method provided the sequence of the first 40 amino acids for both proteins. The putative nucleic acid binding site found in several type C viral nucleoproteins was contained within this sequence, with the most homology centered around an eight-amino acid region involving seven identical residues and one substitution. Antisera were developed in rabbits, and specificity and titers were determined by electroblotting and immunoautoradiography. By this technique, an immunological cross-reaction was found between BLV p12 and FeLV p10. The shared antigenic determinant most likely exists in the highly conserved eight-amino acid region. Although this sequence is also highly conserved in the nucleic acid-binding proteins of murine leukemia viruses, the shared antigenic determinant is not found in these or any other type C viruses tested. It is suggested that substitution of arginine (BLV p12/FeLV p10) to lysine (murine leukemia virus p10) is sufficient to elicit a change in antibody specificity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Coleman J. E. Physiochemical properties of DNA binding proteins: gene 32 protein of T4 and Escherichia coli unwinding protein. Biochemistry. 1975 Dec 16;14(25):5485–5491. doi: 10.1021/bi00696a017. [DOI] [PubMed] [Google Scholar]
- Anderson R. A., Nakashima Y., Coleman J. E. Chemical modifications of functional residues of fd gene 5 DNA-binding protein. Biochemistry. 1975 Mar 11;14(5):907–917. doi: 10.1021/bi00676a006. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Noyes A. N., Boyer M. L., Marr K. Hemoglobin 3 chains in apes. Primary structures and the presumptive nature of back mutation in a normally silent gene. J Biol Chem. 1973 Feb 10;248(3):992–1003. [PubMed] [Google Scholar]
- Ferrer J. F. Bovine leukosis: natural transmission and principles of control. J Am Vet Med Assoc. 1979 Dec 15;175(12):1281–1286. [PubMed] [Google Scholar]
- Ferrer J. F., Kenyon S. J., Gupta P. Milk of dairy cows frequently contains a leukemogenic virus. Science. 1981 Aug 28;213(4511):1014–1016. doi: 10.1126/science.6267692. [DOI] [PubMed] [Google Scholar]
- Gupta P., Ferrer J. F. Detection of a precursor-like protein of bovine leukaemia virus structural polypeptides in purified virions. J Gen Virol. 1980 Apr;47(2):311–322. doi: 10.1099/0022-1317-47-2-311. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Oroszlan S. Separation of amino acid phenylthiohydantoins by high-performance liquid chromatography on phenylalkyl support. Anal Biochem. 1980 Feb;102(1):1–7. doi: 10.1016/0003-2697(80)90307-3. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Smythers G. W., Marquardt H., Oroszlan S. Amino-terminal amino acid sequence and carboxyl-terminal analysis of Rauscher murine leukemia virus glycoproteins. Virology. 1978 Mar;85(1):319–322. doi: 10.1016/0042-6822(78)90437-3. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Jarrett O. Virology and host range of feline leukemia virus. J Am Vet Med Assoc. 1971 Mar 15;158(6 Suppl):1032+–1032+. [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Long C. W., Henderson L. E., Oroszlan S. Isolation and characterization of low-molecular-weight DNA-binding proteins from retroviruses. Virology. 1980 Jul 30;104(2):491–496. doi: 10.1016/0042-6822(80)90352-9. [DOI] [PubMed] [Google Scholar]
- Mammerickx M., Portetelle D., Burny A. Experimental cross-transmissions of bovine leukemia virus (BLV) between several animal species. Zentralbl Veterinarmed B. 1981;28(1):69–81. doi: 10.1111/j.1439-0450.1981.tb01740.x. [DOI] [PubMed] [Google Scholar]
- McDonald H. C., Ferrer J. F. Detection, quantitation, and characterization of the major internal virion antigen of the bovine leukemia virus by radioimmunoassay. J Natl Cancer Inst. 1976 Oct;57(4):875–882. doi: 10.1093/jnci/57.4.875. [DOI] [PubMed] [Google Scholar]
- McDonald H. C., Graves D. C., Ferrer J. F. Isolation and characterization of an antigen of the bovine C-type virus. Cancer Res. 1976 Apr;36(4):1251–1257. [PubMed] [Google Scholar]
- Olpin J. L., Oroszlan S. Rapid stepwise solubilization and purification of type C retrovirus structural proteins by extraction with organic solvent. Anal Biochem. 1980 Apr;103(2):331–336. doi: 10.1016/0003-2697(80)90619-3. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T. D., Henderson L. E., Stephenson J. R., Gilden R. V. Amino-terminal sequence of bovine leukemia virus major internal protein: homology with mammalian type C virus p30 structural proteins. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2996–3000. doi: 10.1073/pnas.76.6.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Sarngadharan M. G., Copeland T. D., Kalyanaraman V. S., Gilden R. V., Gallo R. C. Primary structure analysis of the major internal protein p24 of human type C T-cell leukemia virus. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1291–1294. doi: 10.1073/pnas.79.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. A transmissible virus-induced lymphocytic leukemia of the cat. J Natl Cancer Inst. 1969 Jun;42(6):987–1014. [PubMed] [Google Scholar]
- Rickard C. G., Post J. E., Noronha F., Barr L. M. Interspecies infection by feline leukemia virus: serial cell-free transmission in dogs of malignant lymphomas induced by feline leukemia virus. Bibl Haematol. 1973;39:102–112. doi: 10.1159/000427807. [DOI] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Van Der Maaten M. J., Miller J. M., Boothe A. D. Replicating type-C virus particles in monolayer cell cultures of tissues from cattle with lymphosarcoma. J Natl Cancer Inst. 1974 Feb;52(2):491–497. doi: 10.1093/jnci/52.2.491. [DOI] [PubMed] [Google Scholar]