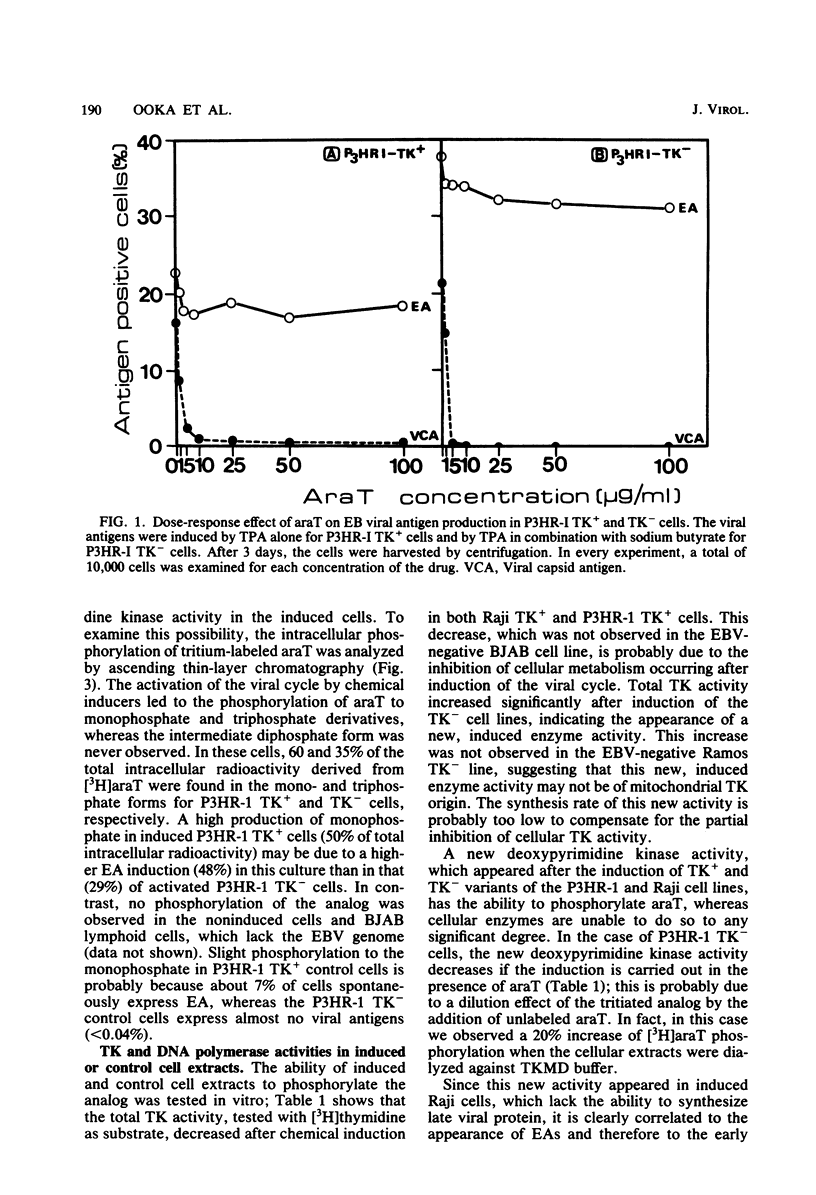
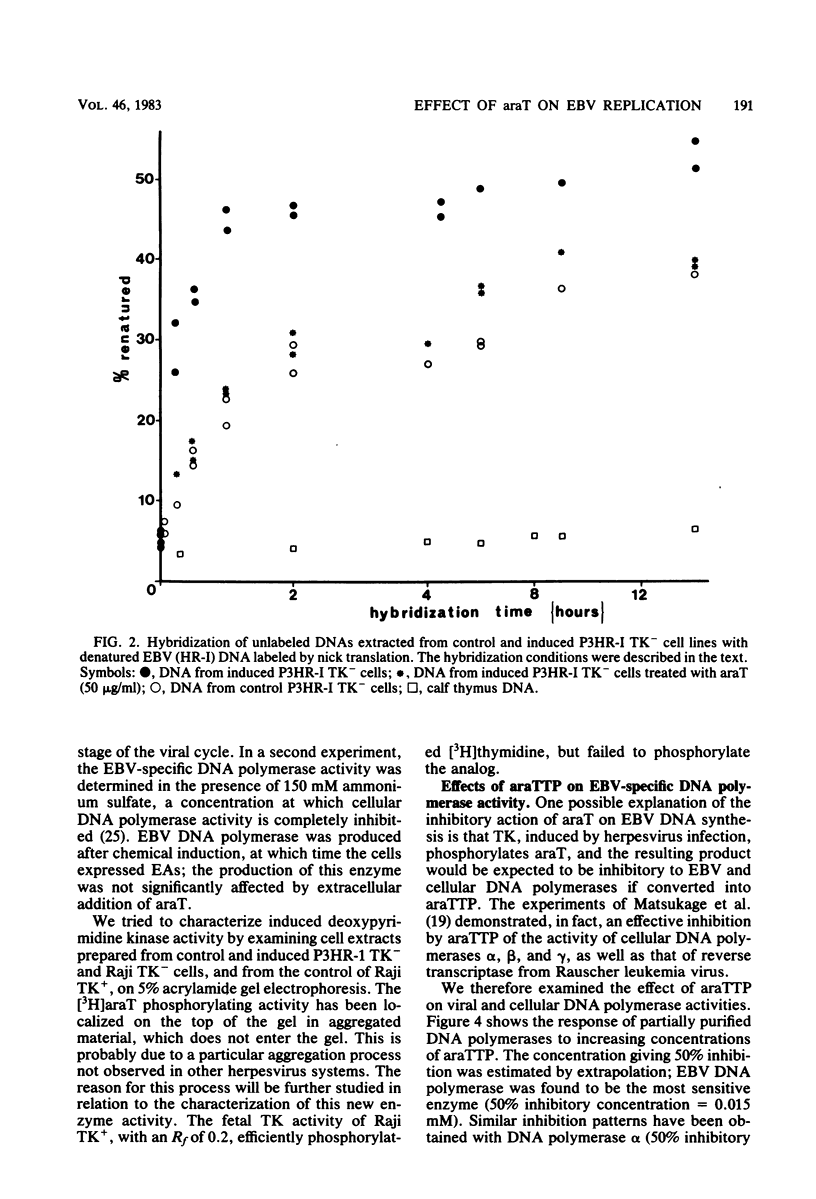
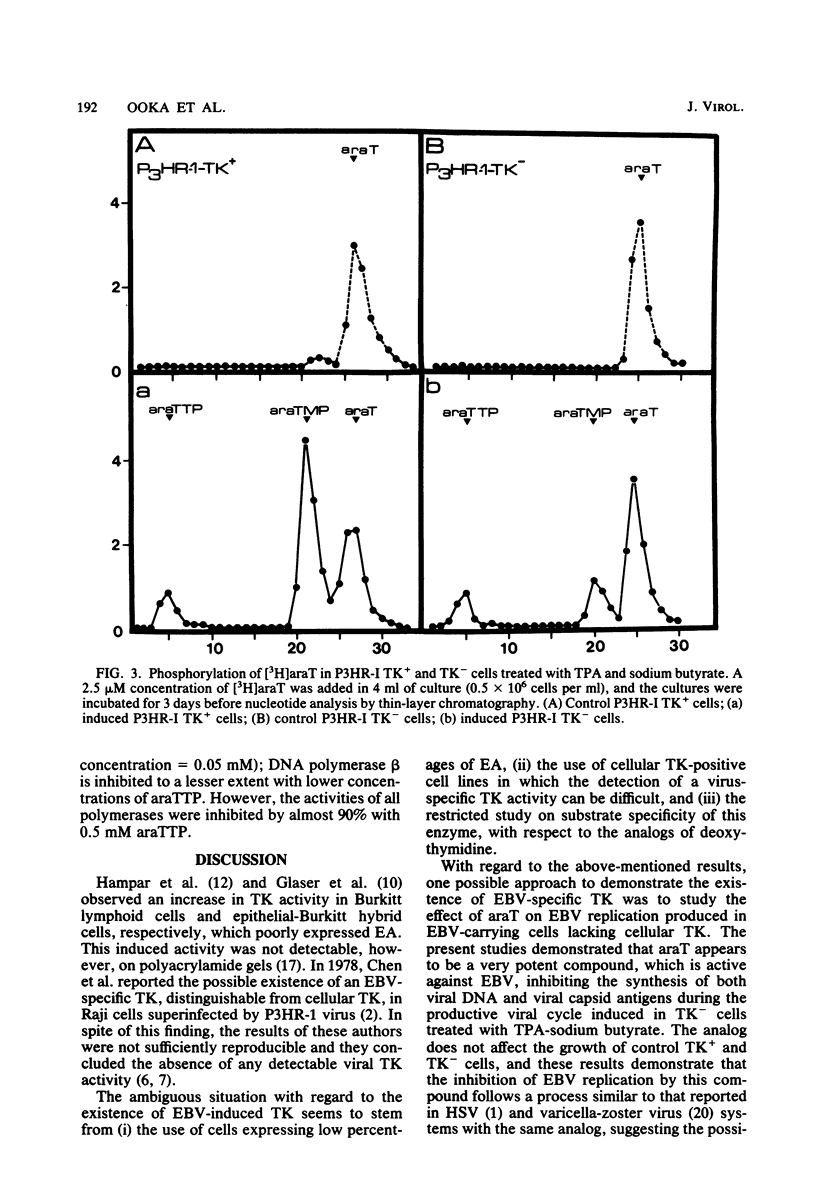
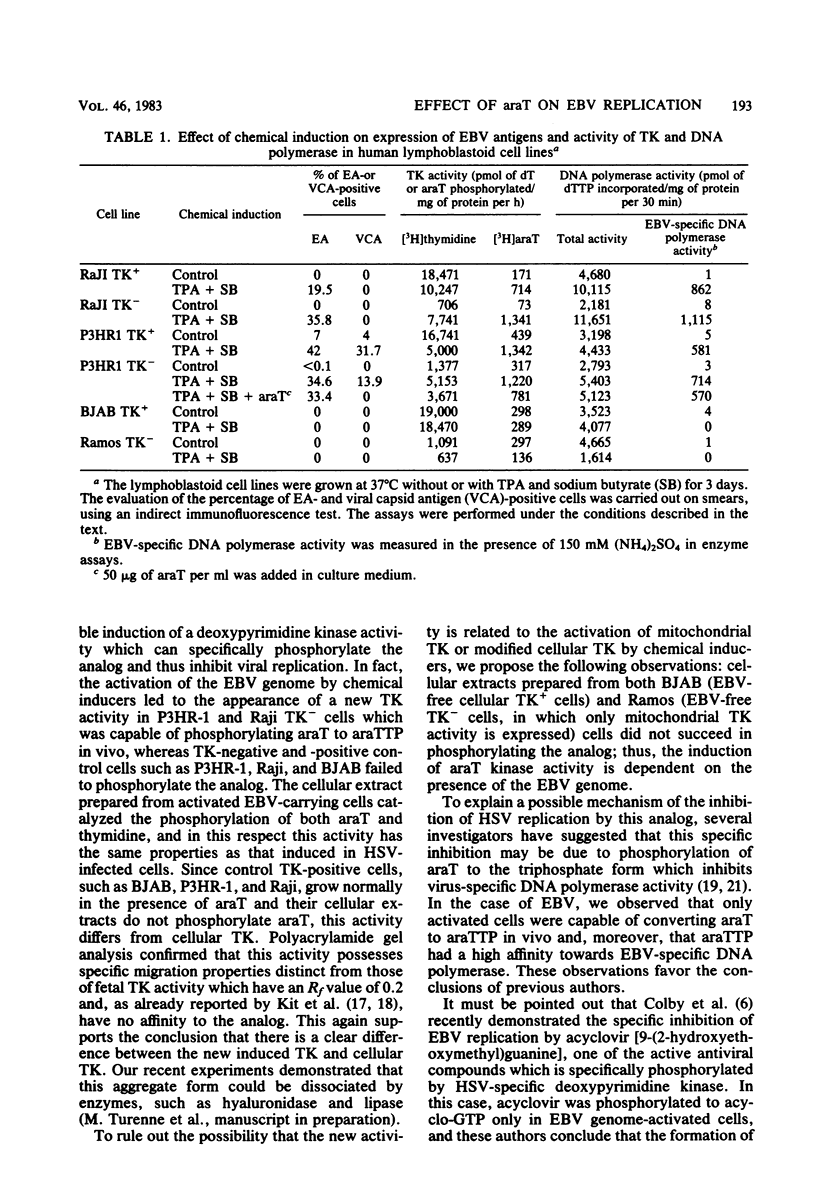
Abstract
1-beta-D-Arabinofuranosylthymine (araT) is a selective inhibitor of Epstein-Barr virus replication induced in both thymidine kinase (TK)-negative (TK-) and TK+ variants of the lymphoid cell line P3HR-I. This analog has no effect on the growth of noninduced cells (T. Ooka and A. Calender, Virology 104:219-223, 1980). The synthesis of early antigens is not affected by the analog, whereas that of late viral capsid antigens is completely inhibited, as demonstrated by the indirect immunofluorescence technique; kinetic reassociation experiments have also shown that araT strongly inhibits replication of viral DNA. Phosphorylation of the tritiated form of the analog ([3H]araT) was analyzed by thin-layer chromatography in cultures of control and induced cells, and the results demonstrated that only induced cells can convert the analog to the triphosphate form. These results indicate that the selective effect of araT in induced cells is probably related to a new virally induced TK activity. Preliminary characterization of this new activity has shown that it is able to phosphorylate the analog specifically, whereas cellular TKs cannot. araTTP, a final phosphorylation product of araT, is a potent inhibitor of Epstein-Barr virus-specific DNA polymerase, suggesting a possible inhibitory action of this product on Epstein-Barr virus replication.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Chen J. Y., Hoffmann P. J., Glaser R. Studies on the activity of DNase associated with the replication of the Epstein-Barr virus. Virology. 1980 Jan 30;100(2):334–338. doi: 10.1016/0042-6822(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Clough W. Deoxyribonuclease activity found in Epstein--Barr virus producing lymphoblastoid cells. Biochemistry. 1979 Oct 16;18(21):4517–4521. doi: 10.1021/bi00588a009. [DOI] [PubMed] [Google Scholar]
- Colby B. M., Furman P. A., Shaw J. E., Elion G. B., Pagano J. S. Phosphorylation of acyclovir [9-(2-hydroxyethoxymethyl)guanine] in Epstein-Barr virus-infected lymphoblastoid cell lines. J Virol. 1981 May;38(2):606–611. doi: 10.1128/jvi.38.2.606-611.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Ogino T., Zimmerman J., Jr, Rapp F. Thymidine kinase activity in Burkitt lymphoblastoid somatic cell hybrids after induction of the EB virus. Proc Soc Exp Biol Med. 1973 Apr;142(4):1059–1062. doi: 10.3181/00379727-142-37176. [DOI] [PubMed] [Google Scholar]
- Grossberger D., Clough W. Characterization of purified Epstein--Barr virus induced deoxyribonucleic acid polymerase: nucleotide turnover, processiveness, and phosphonoacetic acid sensitivity. Biochemistry. 1981 Jul 7;20(14):4049–4055. doi: 10.1021/bi00517a016. [DOI] [PubMed] [Google Scholar]
- Hampar B., Derge J. G., Martos L. M., Walker J. L. Synthesis of Epstein-Barr virus after activation of the viral genome in a "virus-negative" human lymphoblastoid cell (Raji) made resistant to 5-bromodeoxyuridine (thymidine kinase-virus antigen-immunofluorescence-herpesvirus fingerprints). Proc Natl Acad Sci U S A. 1972 Jan;69(1):78–82. doi: 10.1073/pnas.69.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. J., Cheng Y. C. The deoxyribonuclease induced after infection of KB cells by herpes simplex virus type 1 or type 2. I. Purification and characterization of the enzyme. J Biol Chem. 1978 May 25;253(10):3557–3562. [PubMed] [Google Scholar]
- Keir H. M., Subak-Sharpe H., Shedden W. I., Watson D. H., Wildy P. Immunological evidence for a specific DNA polymerase produced after infection by herpes simplex virus. Virology. 1966 Sep;30(1):154–157. doi: 10.1016/s0042-6822(66)81022-x. [DOI] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G. N., Trkula D., Dubbs D. R. Viral-induced thymidine kinase isozymes. Prog Med Virol. 1975;21:13–34. [PubMed] [Google Scholar]
- Kit S., Leung W. C., Kaplan L. A. Distinctive molecular forms of thymidine kinase in mitochondria of normal and bromodeoxyuridine-resistant HeLa cells. Eur J Biochem. 1973 Nov 1;39(1):43–48. doi: 10.1111/j.1432-1033.1973.tb03101.x. [DOI] [PubMed] [Google Scholar]
- Matsukage A., Ono K., Ohashi A., Takahashi T., Nakayama C., Saneyoshi M. Inhibitory effect of 1-beta-D-arabinofuranosylthymine 5'-triphosphate and 1-beta-D-arabinofuranosylcytosine 5'-triphosphate on DNA polymerases from murine cells and oncornavirus. Cancer Res. 1978 Sep;38(9):3076–3079. [PubMed] [Google Scholar]
- Miller R. L., Iltis J. P., Rapp F. Differential effect of arabinofuranosylthymine of the replication of human herpesviruses. J Virol. 1977 Sep;23(3):679–684. doi: 10.1128/jvi.23.3.679-684.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Arendes F., Falke D. Phosphorylation of arabinofuranosylthymine in non-infected and herpesvirus (TK+ and TK-)-infected cells. J Gen Virol. 1979 May;43(2):261–271. doi: 10.1099/0022-1317-43-2-261. [DOI] [PubMed] [Google Scholar]
- Ogino T., Otsuka T., Takahashi M. Induction of deoxypyrimidine kinase activity in human embryonic lung cells infected with varicella-zoster virus. J Virol. 1977 Mar;21(3):1232–1235. doi: 10.1128/jvi.21.3.1232-1235.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooka T., Calender A. Effects of arabinofuranosylthymine on Epstein-Barr virus replication. Virology. 1980 Jul 15;104(1):219–223. doi: 10.1016/0042-6822(80)90379-7. [DOI] [PubMed] [Google Scholar]
- Ooka T., Daillie J. Temperature dependent phosphorylation of [3H]thymidine and its incorporation into DNA by KB cells. Exp Cell Res. 1977 Feb;104(2):319–324. doi: 10.1016/0014-4827(77)90097-0. [DOI] [PubMed] [Google Scholar]
- Ooka T., Lenoir G., Daillie J. Characterization of an Epstein-Barr virus-induced DNA polymerase. J Virol. 1979 Jan;29(1):1–10. doi: 10.1128/jvi.29.1.1-10.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIZER L. I., COHEN S. S. Metabolism of pyrimidine arabinonucleosides and cyclonucleosides in Escherichia coli. J Biol Chem. 1960 Aug;235:2387–2392. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roubal J., Klein G. Synthesis of thymidine kinase (TK) in Epstein-Barr virus-superinfected Raji TK-negative cells. Intervirology. 1981;15(1):43–48. doi: 10.1159/000149213. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]



