Abstract
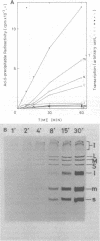
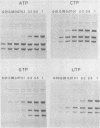
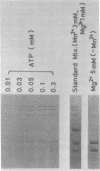
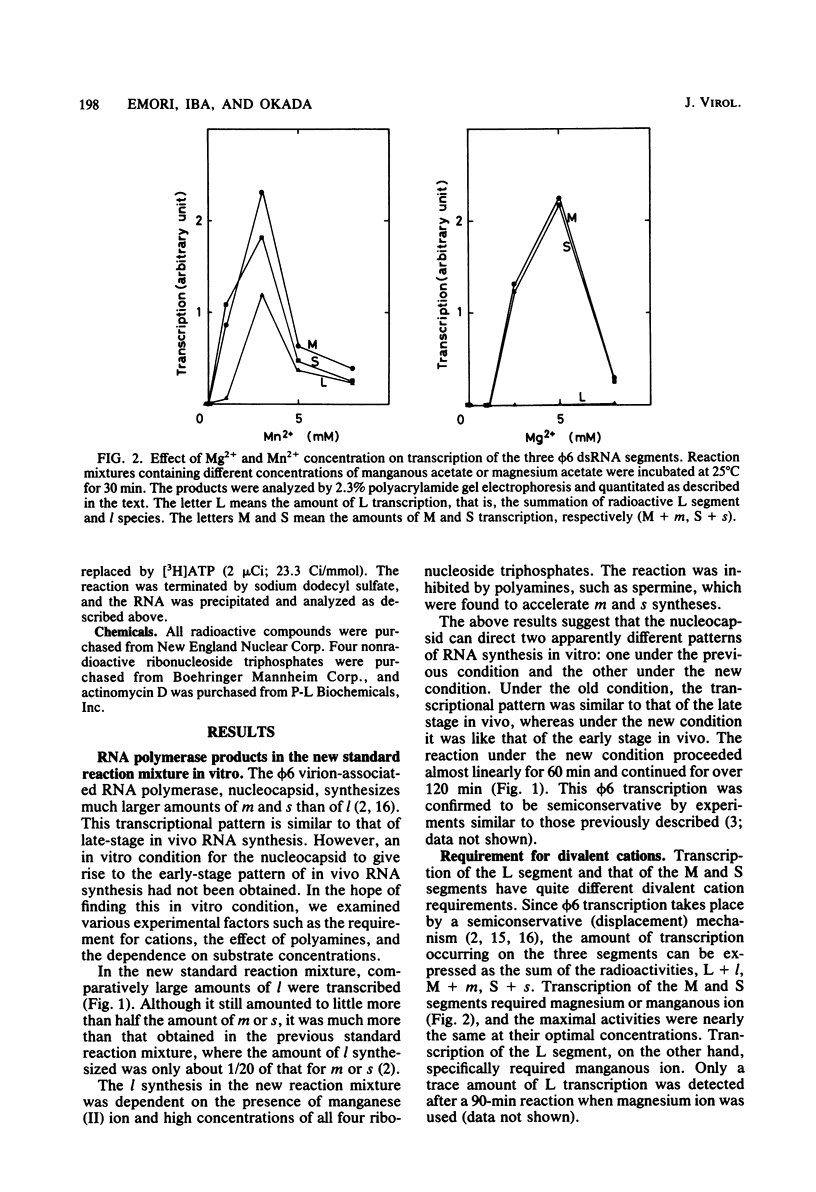
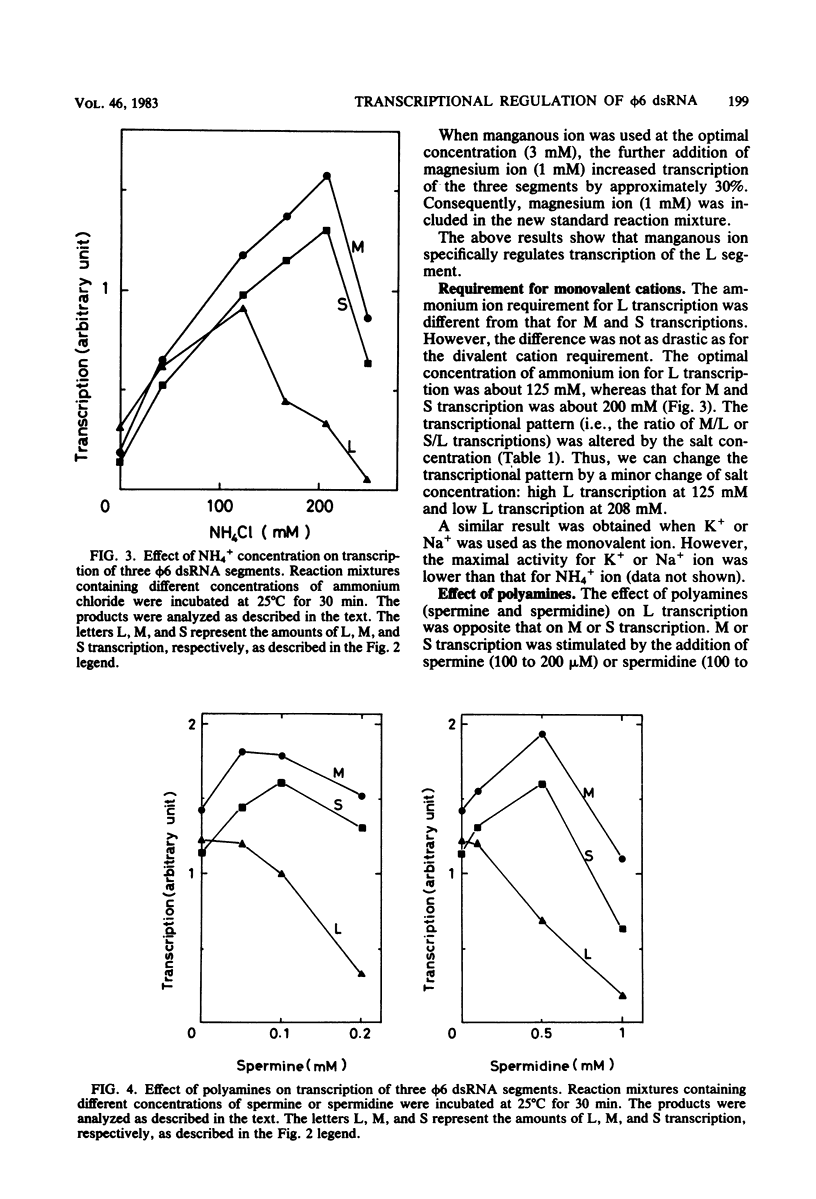
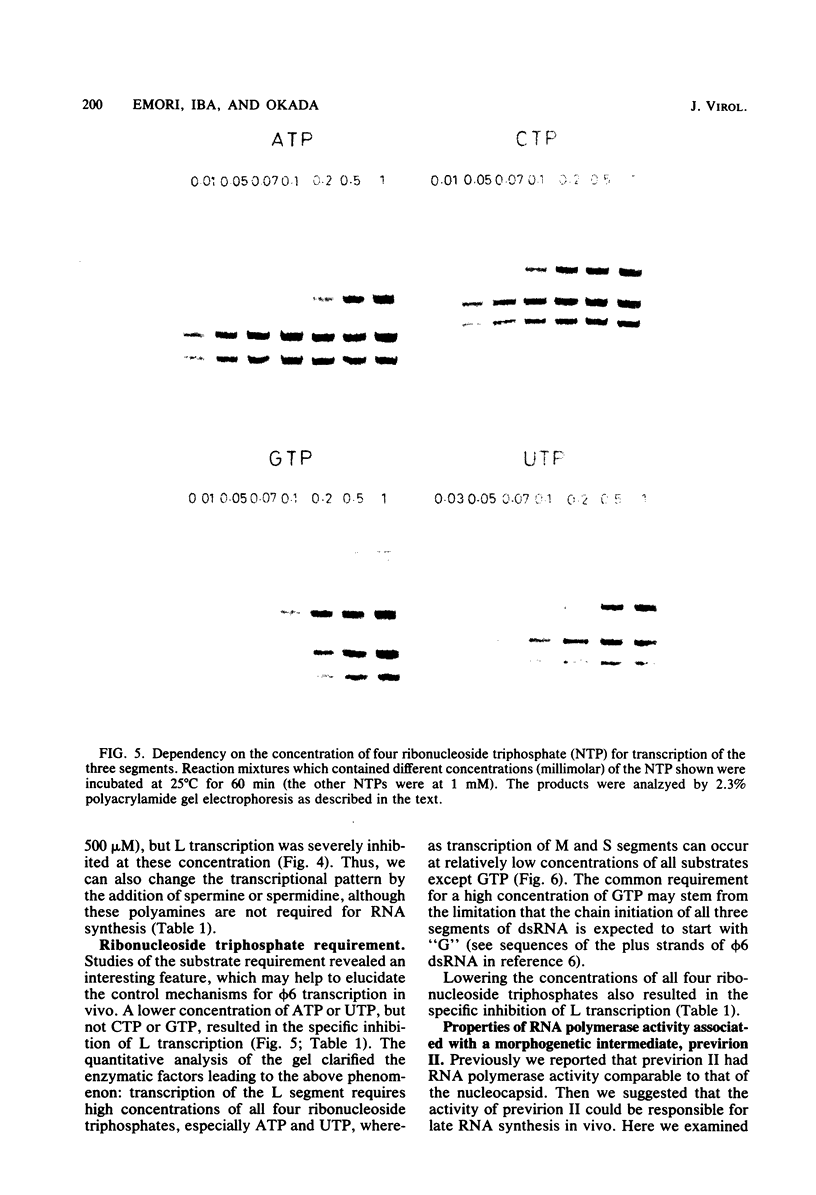
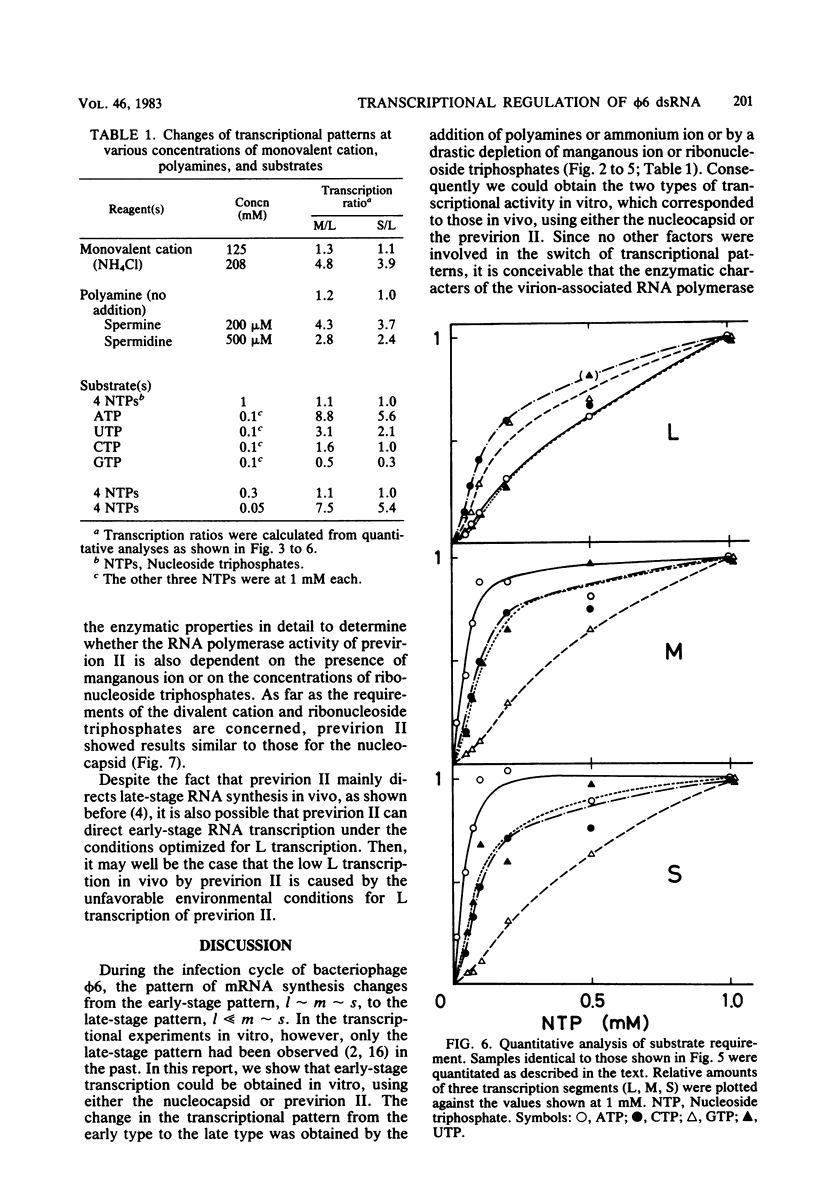
Three double-stranded RNA segments of bacteriophage phi 6 (L, M, and S) were transcribed in vitro by a virion-associated RNA polymerase. Regulation of L transcription was distinct from regulation of M and S transcription. Transcription of the L segment, which codes for early proteins, required manganous ion and high concentrations of all four ribonucleoside triphosphates and was inhibited by polyamines such as spermine. Transcription of the M and S segments, which code for late proteins, required manganous or magnesium ion and relatively low concentrations of all ribonucleoside triphosphates except GTP and was enhanced by polyamines. Optimal conditions for L transcription were more stringent than those for M and S transcription. These two apparently different patterns produced in in vitro transcription presumably reflect the two distinct in vivo transcription patterns; i.e., (i) similar amounts of three single-stranded RNA species were transcribed from the three corresponding segments of double-stranded RNA (early pattern) and (ii) a much larger amount of single-stranded RNA species was transcribed from M and S segments than from the L segment (late pattern). The early transcription pattern may be changed into the late pattern by a change of environment, such as substrate concentration. This suggests that the different enzymatic properties under the different environmental conditions of the virion-associated transcriptase are responsible for the transcriptional regulation throughout the infection cycle of bacteriophage phi 6.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuppels D. A., Van Etten J. L., Burbank D. E., Lane L. C., Vidaver A. K. In vitro translation of the three bacteriophage phi 6 RNAs. J Virol. 1980 Jul;35(1):249–251. doi: 10.1128/jvi.35.1.249-251.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Assignment of viral proteins to the three double-stranded RNA segments of bacteriophage phi 6 genome: translation of phi 6 messenger RNAs transcribed in vitro. Mol Gen Genet. 1980;180(2):385–389. doi: 10.1007/BF00425852. [DOI] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Morphogenetic pathway of bacteriophage phi 6. A flow analysis of subviral and viral particles in infected cells. J Mol Biol. 1982 Jan 15;154(2):287–310. doi: 10.1016/0022-2836(82)90065-1. [DOI] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Semi-conservative transcription of double-stranded RNA catalyzed by bacteriophage phi 6 RNA polymerase. J Biochem. 1980 Dec;88(6):1569–1575. doi: 10.1093/oxfordjournals.jbchem.a133131. [DOI] [PubMed] [Google Scholar]
- Etten J. V., Lane L., Gonzalez C., Partridge J., Vidaver A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J Virol. 1976 May;18(2):652–658. doi: 10.1128/jvi.18.2.652-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iba H., Nanno M., Okada Y. Identification and partial purification of a lytic enzyme in the bacteriophage phi 6 virion. FEBS Lett. 1979 Jul 15;103(2):234–237. doi: 10.1016/0014-5793(79)81334-4. [DOI] [PubMed] [Google Scholar]
- Iba H., Watanabe T., Emori Y., Okada Y. Three double-stranded RNA genome segments of bacteriophage phi 6 have homologous terminal sequences. FEBS Lett. 1982 May 3;141(1):111–115. doi: 10.1016/0014-5793(82)80027-6. [DOI] [PubMed] [Google Scholar]
- Kakitani H., Iba H., Okada Y. Penetration and partial uncoating of bacteriophage phi 6 particle. Virology. 1980 Mar;101(2):475–483. doi: 10.1016/0042-6822(80)90461-4. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Furuichi Y. A unique class of compound, guanosine-nucleoside tetraphosphate G(5')pppp(5')N, synthesized during the in vitro transcription of cytoplasmic polyhedrosis virus of Bombyx mori. Structural determination and mechanism of formation. J Biol Chem. 1982 Jan 10;257(1):485–494. [PubMed] [Google Scholar]
- Ulmanen I., Broni B. A., Krug R. M. Role of two of the influenza virus core P proteins in recognizing cap 1 structures (m7GpppNm) on RNAs and in initiating viral RNA transcription. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7355–7359. doi: 10.1073/pnas.78.12.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usala S. J., Brownstein B. H., Haselkorn R. Displacement of parental RNA strands during in vitro transcription by bacteriophage phi 6 nucleocapsids. Cell. 1980 Apr;19(4):855–862. doi: 10.1016/0092-8674(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Vidaver A. K., Koski R. K., Burnett J. P. Base composition and hybridization studies of the three double-stranded RNA segments of bacteriophage phi 6. J Virol. 1974 Jun;13(6):1254–1262. doi: 10.1128/jvi.13.6.1254-1262.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M., Furuichi Y., Nakashima K., LaFiandra A. J., Shatkin A. J. Excess synthesis of viral mRNA 5-terminal oligonucleotides by reovirus transcriptase. J Biol Chem. 1981 Jun 25;256(12):6507–6514. [PubMed] [Google Scholar]