Abstract
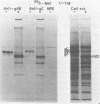
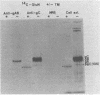
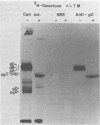
The presence of O-glycosidic linkages on herpes simplex virus type 1 (HSV-1) glycoproteins was indicated by the synthesis and glycosylation of HSV-1 glycoproteins in the presence of tunicamycin. Monospecific antiserum to HSV-1 gC immunoprecipitated a 92,000-molecular-weight protein synthesized in the presence of tunicamycin and isotopically labeled with glucosamine or galactose. Anti-gAB did not immunoprecipitate a carbohydrate-labeled HSV-1 protein synthesized in the presence of tunicamycin. The purified glucosamine-labeled 92,000-molecular-weight protein synthesized in the presence of tunicamycin and the fully glycosylated forms of gAB and gC were tested for their sensitivity to mild alkaline hydrolysis. Purified gAB was resistant to mild alkaline hydrolysis, whereas gC and the 92,000-molecular-weight protein were both sensitive to mild alkaline hydrolysis. These results suggest that O-glycosidic linkages are associated with the HSV-1 gC glycoprotein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. I. Composition, glycopeptide isolation, and structure of the asparagine-linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7260–7269. [PubMed] [Google Scholar]
- Baenziger J., Kornfeld S. Structure of the carbohydrate units of IgA1 immunoglobulin. II. Structure of the O-glycosidically linked oligosaccharide units. J Biol Chem. 1974 Nov 25;249(22):7270–7281. [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M., Osawa T. Isolation and characterization of a glycoprotein from human group O erythrocyte membrane. J Biol Chem. 1973 Jul 25;248(14):5100–5105. [PubMed] [Google Scholar]
- Gahmberg C. G., Jokinen M., Karhi K. K., Andersson L. C. Effect of tunicamycin on the biosynthesis of the major human red cell sialoglycoprotein, glycophorin A, in the leukemia cell line K562. J Biol Chem. 1980 Mar 10;255(5):2169–2175. [PubMed] [Google Scholar]
- Gibson W., Roizman B. Proteins specified by herpes simplex virus. Staining and radiolabeling properties of B capsid and virion proteins in polyacrylamide gels. J Virol. 1974 Jan;13(1):155–165. doi: 10.1128/jvi.13.1.155-165.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Heifetz A., Keenan R. W., Elbein A. D. Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 1979 May 29;18(11):2186–2192. doi: 10.1021/bi00578a008. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Honess R. W., Cassai E., Roizman B. Proteins specified by herpes simplex virus. XII. The virion polypeptides of type 1 strains. J Virol. 1974 Sep;14(3):640–651. doi: 10.1128/jvi.14.3.640-651.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Katz E., Margalith E., Duksin D. Antiviral activity of tunicamycin on herpes simplex virus. Antimicrob Agents Chemother. 1980 Jun;17(6):1014–1022. doi: 10.1128/aac.17.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E., Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. I. Structure of the lipid-linked oligosaccharide precursor of the complex-type oligosaccharides of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7762–7770. [PubMed] [Google Scholar]
- Niemann H., Klenk H. D. Glycoprotein E1 of coronavirus A59: a new type of viral glycoprotein. Adv Exp Med Biol. 1981;142:119–131. doi: 10.1007/978-1-4757-0456-3_10. [DOI] [PubMed] [Google Scholar]
- Nilsson B., Nordén N. E., Svensson S. Structural studies on the carbohydrate portion of fetuin. J Biol Chem. 1979 Jun 10;254(11):4545–4553. [PubMed] [Google Scholar]
- Olofsson S., Blomberg J., Lycke E. O-glycosidic carbohydrate-peptide linkages of Herpes simplex virus glycoproteins. Arch Virol. 1981;70(4):321–329. doi: 10.1007/BF01320247. [DOI] [PubMed] [Google Scholar]
- Olofsson S., Jeansson S., Lycke E. Unusual lectin-binding properties of a herpes simplex virus type 1-specific glycoprotein. J Virol. 1981 May;38(2):564–570. doi: 10.1128/jvi.38.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake M. L., Nystrom P., Pizer L. I. Herpesvirus glycoprotein synthesis and insertion into plasma membranes. J Virol. 1982 May;42(2):678–690. doi: 10.1128/jvi.42.2.678-690.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Courtney R. J. Polypeptide synthesized in herpes simplex virus type 2-infected HEp-2 cells. Virology. 1975 Jul;66(1):217–228. doi: 10.1016/0042-6822(75)90192-0. [DOI] [PubMed] [Google Scholar]
- Shida H., Dales S. Biogenesis of vaccinia: carbohydrate of the hemagglutinin molecules. Virology. 1981 May;111(1):56–72. doi: 10.1016/0042-6822(81)90653-x. [DOI] [PubMed] [Google Scholar]
- Spiro R. G., Bhoyroo V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J Biol Chem. 1974 Sep 25;249(18):5704–5717. [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]