Abstract
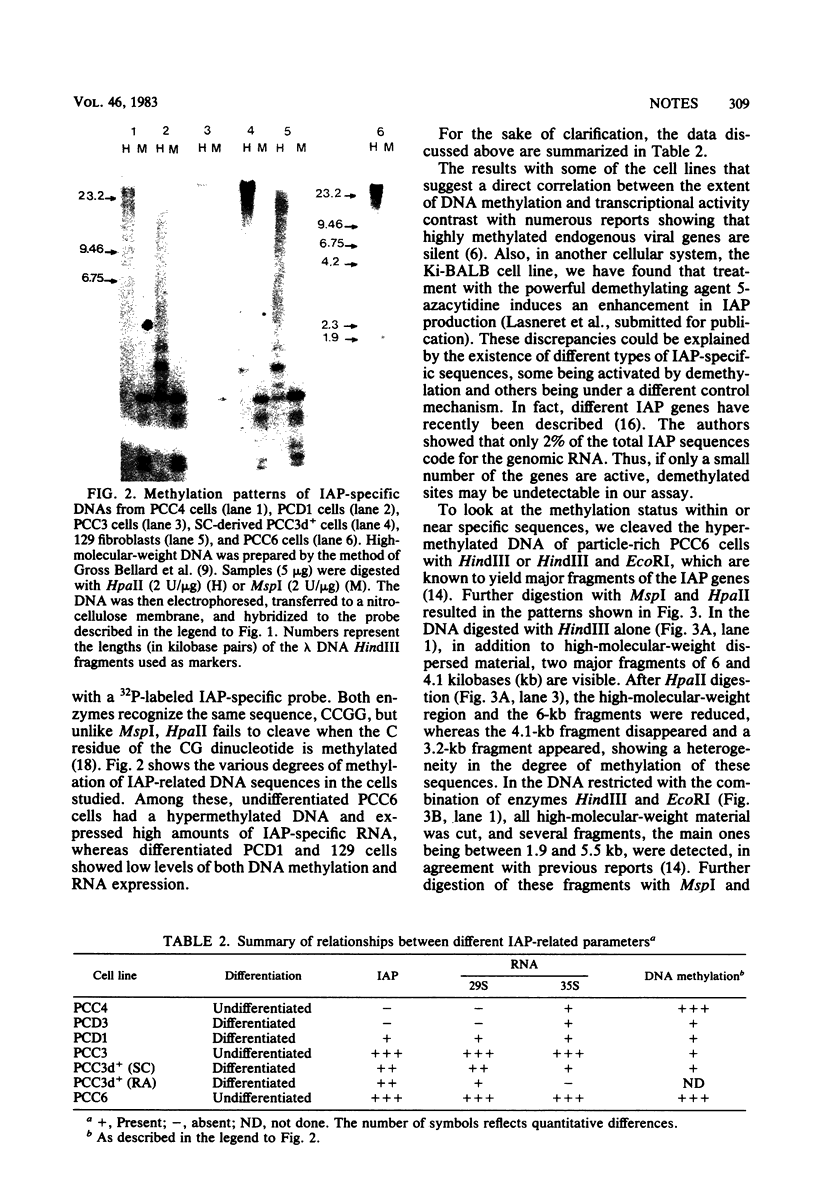
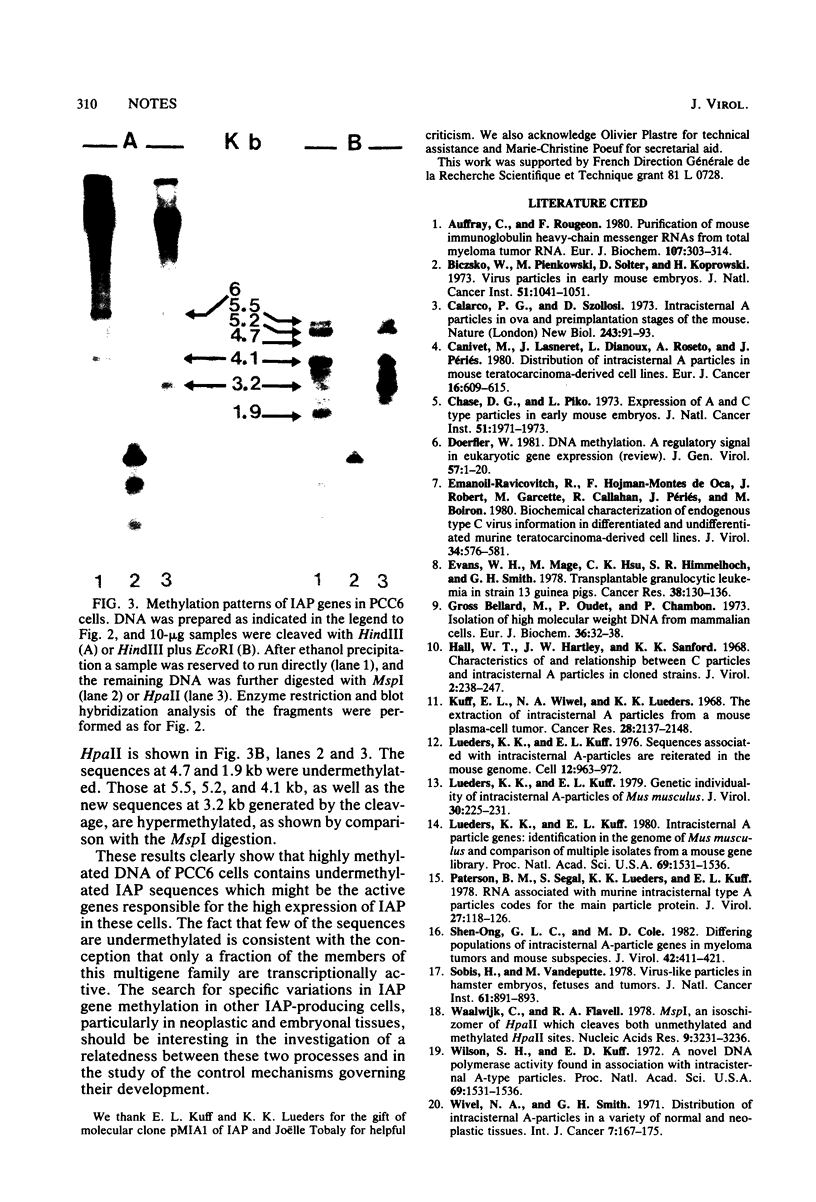
Two main intracisternal-A-particle-specific RNA species (29 to 30S and 35 to 36S) are variably expressed in particle-producing and particle-nonproducing murine teratocarcinoma cell lines. An analysis of DNA methylation patterns after hybridization with an intracisternal-A-particle-specific probe showed an apparent direct correlation between DNA methylation and RNA expression. However, when the methylation assay was performed on the excised intracisternal A particle genes in one of the particle-rich cell lines (PCC6), some undermethylated sequences were detectable. These results are consistent with the concept that only few of the genes are transcriptionally active.
Full text
PDF
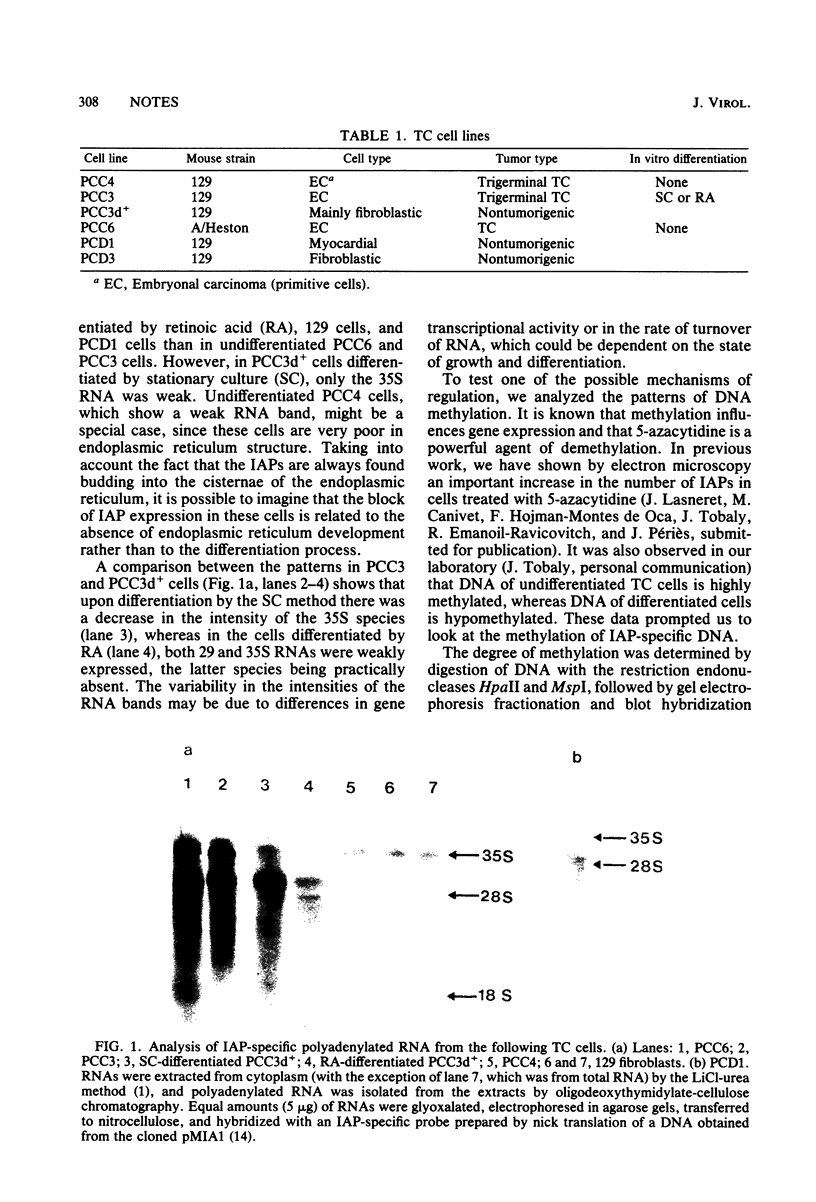
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Biczysko W., Pienkowski M., Solter D., Koprowski H. Virus particles in early mouse embryos. J Natl Cancer Inst. 1973 Sep;51(3):1041–1050. doi: 10.1093/jnci/51.3.1041. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Szollosi D. Intracisternal A particles in ova and preimplantation stages of the mouse. Nat New Biol. 1973 May 16;243(124):91–93. [PubMed] [Google Scholar]
- Canivet M., Lasneret J., Dianoux L., Roseto A., Peries J. Distribution of intracisternal A particles in mouse teratocarcinoma-derived cell lines. Eur J Cancer. 1980 May;16(5):609–615. doi: 10.1016/0014-2964(80)90200-5. [DOI] [PubMed] [Google Scholar]
- Chase D. G., Pikó L. Expression of A- and C-type particles in early mouse embryos. J Natl Cancer Inst. 1973 Dec;51(6):1971–1975. doi: 10.1093/jnci/51.6.1971. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation--a regulatory signal in eukaryotic gene expression. J Gen Virol. 1981 Nov;57(Pt 1):1–20. doi: 10.1099/0022-1317-57-1-1. [DOI] [PubMed] [Google Scholar]
- Emanoil-Ravicovitch R., Hojman-Montes-de-Oca F., Robert J., Garcette M., Callahan R., Peries J., Boiron M. Biochemical characterization of endogenous type C virus information in differentiated and undifferentiated murine teratocarcinoma-derived cell lines. J Virol. 1980 May;34(2):576–581. doi: 10.1128/jvi.34.2.576-581.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Mage M. G., Hsu C. K., Himmelhoch S. R., Smith G. H. Transplantable granulocytic leukemia in strain 13 guinea pigs. Cancer Res. 1978 Jan;38(1):130–136. [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hall W. T., Hartley J. W., Sanford K. K. Characteristics of and relationship between C particles and intracisternal A particles in cloned cell strains. J Virol. 1968 Mar;2(3):238–247. doi: 10.1128/jvi.2.3.238-247.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuff E. L., Wivel N. A., Lueders K. K. The extraction of intracisternal A-particles from a mouse plasma-cell tumor. Cancer Res. 1968 Oct;28(10):2137–2148. [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Genetic individuality of intracisternal A-particles of Mus musculus. J Virol. 1979 Apr;30(1):225–231. doi: 10.1128/jvi.30.1.225-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Sequences associated with intracisternal A particles are reiterated in the mouse genome. Cell. 1977 Dec;12(4):963–972. doi: 10.1016/0092-8674(77)90161-1. [DOI] [PubMed] [Google Scholar]
- Shen-Ong G. L., Cole M. D. Differing populations of intracisternal A-particle genes in myeloma tumors and mouse subspecies. J Virol. 1982 May;42(2):411–421. doi: 10.1128/jvi.42.2.411-421.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobis H., Vandeputte M. Viruslike particles in hamster embryos, fetuses, and tumors. J Natl Cancer Inst. 1978 Sep;61(3):891–895. [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. H., Kuff E. L. A novel DNA polymerase activity found in association with intracisternal A-type particles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1531–1536. doi: 10.1073/pnas.69.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wivel N. A., Smith G. H. Distribution of intracisternal A-particles in a variety of normal and neoplastic mouse tissues. Int J Cancer. 1971 Jan 15;7(1):167–175. doi: 10.1002/ijc.2910070119. [DOI] [PubMed] [Google Scholar]