Abstract
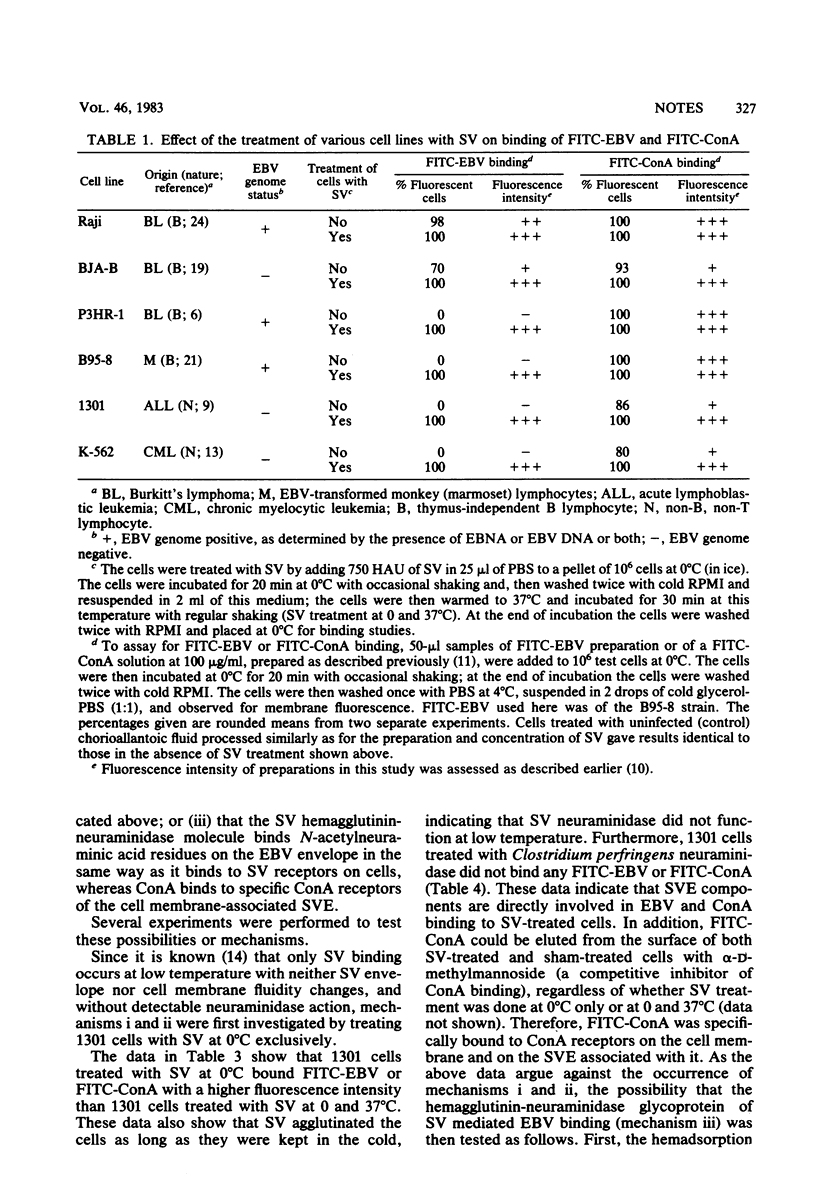
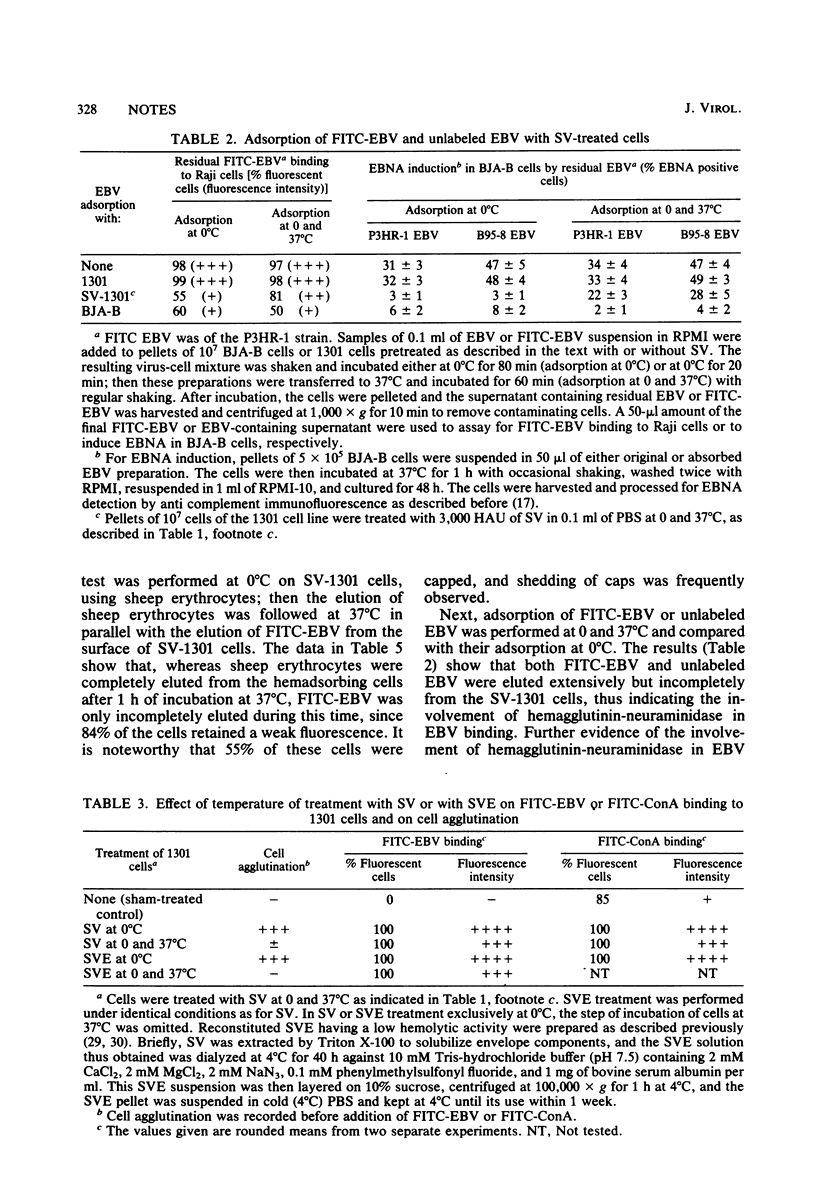
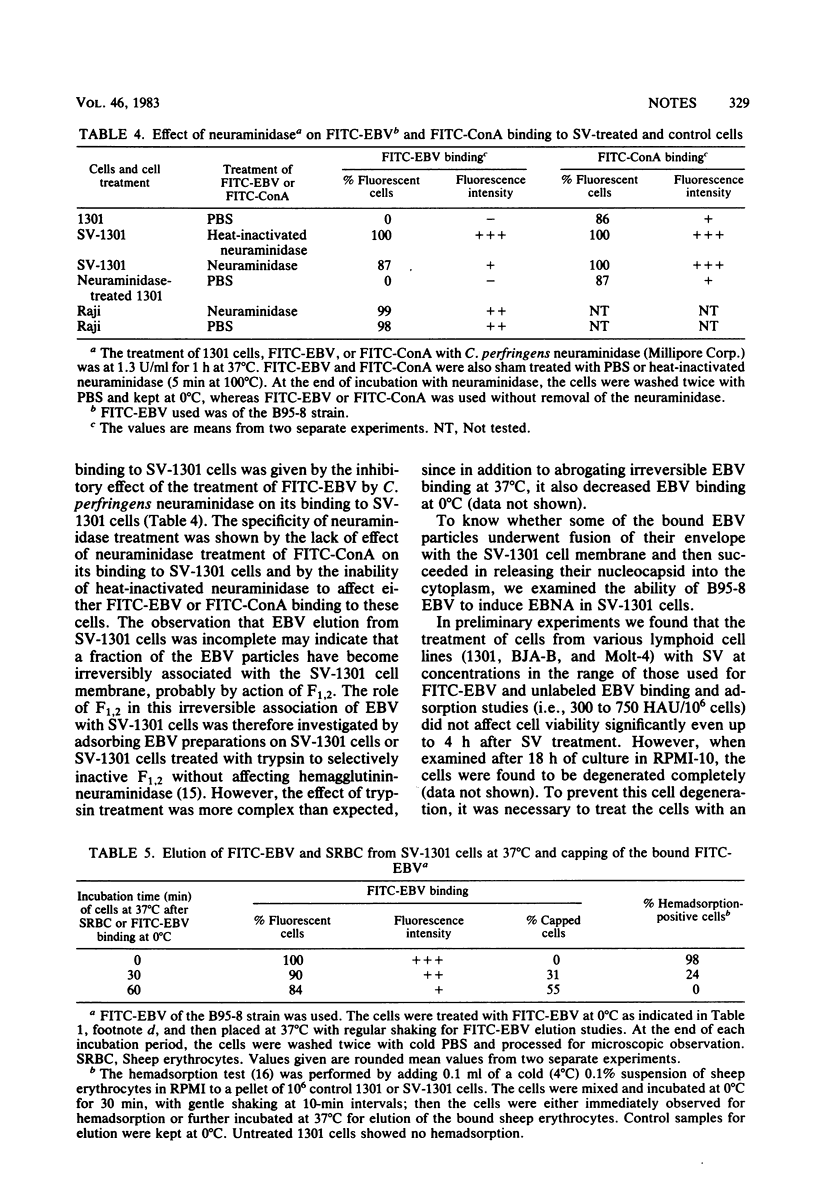
Epstein-Barr virus (EBV) receptor-negative cells were treated with UV-inactivated Sendai virus (SV) or with reconstituted SV envelopes having a low hemolytic activity and then assayed for EBV binding or for susceptibility to EBV infection. EBV binding was assessed by using both unlabeled and fluoresceinated EBV preparations. It was found that SV or SV envelope treatment renders these cells able to bind EBV. Various experiments were performed to clarify the mechanism of this SV-induced binding. The EBV receptor-negative 1301 cells were treated with SV either at 0°C or at both 0 and 37°C successively and then examined for EBV binding at 0°C. It was thus found that when SV treatment was performed exclusively at 0°C, the target cells showed higher fluorescence intensity after their incubation with fluoresceinated EBV. In addition, Clostridium perfringens neuraminidase treatment of 1301 cells did not induce any EBV binding to these cells. These data indicate that EBV binding is not due to the disturbance of the cell membrane by SV envelope fusion or to the uncovering of EBV binding sites on the cells after the enzymatic action of SV neuraminidase. Moreover, bound EBV was partly eluted from SV-treated 1301 cells at 37°C, and the treatment of EBV with C. perfringens neuraminidase inhibited its SV-mediated binding. These data indicate that EBV binds to the hemagglutinin-neuraminidase of SV on the target cell surface and that a fraction of the bound EBV becomes irreversibly associated with the SV-treated cell membrane. Our data also show that EBV can penetrate into 1301 cells which have incorporated SV envelopes into their membrane, as demonstrated by the induction of the EBV-determined nuclear antigen by B95-8 EBV in SV envelope-treated 1301 cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss G. J., Wolf H. An Epstein--Barr virus early protein induces cell fusion. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7162–7165. doi: 10.1073/pnas.78.11.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Bassat H., Mitrani-Rosenbaum S., Goldblum N. Induction of Epstein-Barr virus nuclear antigen and DNA synthesis in a human epithelial cell line after Epstein-Barr virus infection. J Virol. 1982 Feb;41(2):703–708. doi: 10.1128/jvi.41.2.703-708.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Lang C. M., Lee K. J., Schuller D. E., Jacobs D., McQuattie C. Attempt to infect nonmalignant nasopharyngeal epithelial cells from humans and squirrel monkeys with Epstein-Barr virus. J Natl Cancer Inst. 1980 May;64(5):1085–1090. [PubMed] [Google Scholar]
- Glaser R., de Thé G., Lenoir G., Ho J. H. Superinfection epithelial nasopharyngeal carcinoma cells with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):960–963. doi: 10.1073/pnas.73.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Huang D. P., Ho H. C., Ng M. H., Lui M. Possible transformation of nasopharyngeal epithelial cells in culture with Epstein-Barr virus from B95-8 cells. Br J Cancer. 1977 May;35(5):630–634. doi: 10.1038/bjc.1977.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Klein G., Oldstone M. B., Bokish V., Yefenof E. Surface markers on human B and T lymphocytes. VIII. Association between complement and Epstein-Barr virus receptors on human lymphoid cells. Scand J Immunol. 1976;5(4):401–410. doi: 10.1111/j.1365-3083.1976.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khelifa R., Menezes J. Use of fluoresceinated Epstein-Barr virus to study Epstein-Barr virus-lymphoid cell interactions. J Virol. 1982 Feb;41(2):649–656. doi: 10.1128/jvi.41.2.649-656.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khélifa R., Menezes J. Epstein-Barr virus-lymphoid cell interactions. III. Effect of concanavalin A and saccharides on Epstein-Barr virus penetration. J Virol. 1982 May;42(2):402–410. doi: 10.1128/jvi.42.2.402-410.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Giovanella B. C., Lindahl T., Fialkow P. J., Singh S., Stehlin J. S. Direct evidence for the presence of Epstein-Barr virus DNA and nuclear antigen in malignant epithelial cells from patients with poorly differentiated carcinoma of the nasopharynx. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4737–4741. doi: 10.1073/pnas.71.12.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Cytotoxicity of a factor isolated from human spleen. J Natl Cancer Inst. 1973 Feb;50(2):535–538. doi: 10.1093/jnci/50.2.535. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. Kinetics of Sendai virus envelope fusion with erythrocyte membranes and virus-induced hemolysis. Biochemistry. 1979 Nov 13;18(23):5088–5095. doi: 10.1021/bi00590a011. [DOI] [PubMed] [Google Scholar]
- Maeda T., Asano A., Okada Y., Ohnishi S. I. Transmembrane phospholipid motions induced by F glycoprotein in hemagglutinating virus of Japan. J Virol. 1977 Jan;21(1):232–241. doi: 10.1128/jvi.21.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y., Kim J., Koseki I., Mekada E., Shiokawa Y., Okada Y. Modification of cell membranes with viral envelopes during fusion of cells with HVJ (Sendai virus). III. Effects of mono- and di-saccharides on cell fusion and membrane movement of fused cells. Exp Cell Res. 1977 Aug;108(1):95–106. doi: 10.1016/s0014-4827(77)80014-1. [DOI] [PubMed] [Google Scholar]
- Menezes J., Jondal M., Leibold W., Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976 Feb;13(2):303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Menezes J., Seigneurin J. M., Patel P., Bourkas A., Lenoir G. Presence of Epstein-Barr virus receptors, but absence of virus penetration, in cells of an Epstein-Barr virus genome-negative human lymphoblastoid T line (Molt 4). J Virol. 1977 Jun;22(3):816–821. doi: 10.1128/jvi.22.3.816-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonoyama M., Huang C. H., Pagano J. S., Klein G., Singh S. DNA of Epstein-Barr virus detected in tissue of Burkitt's lymphoma and nasopharyngeal carcinoma. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3265–3268. doi: 10.1073/pnas.70.11.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972 Nov;50(2):507–515. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin J. M., Vuillaume M., Lenoir G., De-Thé G. Replication of Epstein-Barr virus: ultrastructural and immunofluorescent studies of P3HR1-superinfected Raji cells. J Virol. 1977 Dec;24(3):836–845. doi: 10.1128/jvi.24.3.836-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro I. M., Klein G., Volsky D. J. Epstein-Barr virus co-reconstituted with Sendai virus envelopes infects Epstein-Barr virus-receptor negative cells. Biochim Biophys Acta. 1981 Aug 5;676(1):19–24. doi: 10.1016/0304-4165(81)90004-0. [DOI] [PubMed] [Google Scholar]
- Siegert W., Mönch T., Valet G. Epstein-Barr virus-induced increase in the concanavalin-A receptor density of established EBV-negative lymphoma lines in vitro. Exp Hematol. 1980 Nov;8(10):1173–1182. [PubMed] [Google Scholar]
- Volsky D. J., Loyter A. An efficient method for reassembly of fusogenic Sendai virus envelopes after solubilization of intact virions with Triton X-100. FEBS Lett. 1978 Aug 15;92(2):190–194. doi: 10.1016/0014-5793(78)80751-0. [DOI] [PubMed] [Google Scholar]
- Volsky D. J., Shapiro I. M., Klein G. Transfer of Epstein-Barr virus receptors to receptor-negative cells permits virus penetration and antigen expression. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5453–5457. doi: 10.1073/pnas.77.9.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H., Klein G., Henle W., Henle G., Clifford P., Santesson L. EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature. 1970 Dec 12;228(5276):1056–1058. doi: 10.1038/2281056a0. [DOI] [PubMed] [Google Scholar]



