Abstract
The results from bacterial strain recovery efforts following hurricanes Katrina and Rita are reported. Over 90% of strains frozen in 10% skim milk were recovered whereas various recovery rates were observed for glycerol-stored stocks (56% and 94% of E. coli, depending upon the laboratory). These observations led to a viability comparison of Streptococcus pyogenes, Campylobacter jejuni, Borrelia burgdorferi, Salmonella enterica subsp. Typhimurium, Pseudomonas aeruginosa and Escherichia coli strains stored in glycerol or skim milk. In all bacteria examined, 10% skim milk resulted in significantly longer viability after thawing than 15% glycerol solutions currently used in most laboratories.
1. Introduction
The freezing of bacterial cultures is a common method of preserving strains in the laboratory. As early as 1913 it was observed that an additive, such as sugar, milk or glycerol, protected bacteria from cell death following repetitive freeze/thaw cycles necessary in most bacteriological research (Keith, 1913). Today glycerol is one of the most common cryoprotective agents utilized. Conventional methods for freezing a culture include suspension of bacterial cultures in 15% glycerol (F.M. Ausubel, 1993, Baker, 1998). Indeed, such methods allow for the long-term viability of strains, if temperature can be maintained and multiple freeze thaw cycles avoided (Gruft et al., 1968, Hollander & Nell, 1954, Linscott & Boak, 1960, Nakamura et al., 1962, Howard, 1956). Alternatively, skim milk, a common freeze-drying protectant (Barbaree et al., 1982), can be used as a freezing solution (Takahashi et al., 1982, Essiain & Flournoy, 1986, Sinha et al., 1974, Hasegawa et al., 1967), as is regularly used in our laboratory to store Pseudomonas aeruginosa. A literature review indicates that the predominant studies of cyroprotectants focus on sensitivity to freeze/thaw cycles (Takahashi et al., 1982, Aulet de Saab et al., 2001) with few comparisons of viability over time at elevated temperature (Gruft et al., 1968, Essiain & Flournoy, 1986). While recovering our bacterial strains from 31 days at ambient temperatures (in this case, sustained temperatures of ∼30°C) due to power loss as a result of hurricanes Katrina and Rita, we observed an increase in recovery of strains that were stored in 10% skim milk versus 15% glycerol. Here we report that use of a 10% skim milk solution (wt/vol) enhanced the viability of several bacterial species after thawing and prolonged incubation at 30°C. These data suggest that a 10% skim milk freezing solution will provide greater protection against loss of viability if a freezer fails and may increase long-term survival of stocks when maintained at -80°C.
2. Materials and Methods
2.1 P. aeruginosa strain storage
The Schurr lab stored P. aeruginosa strains in the -80°C freezer after being cultured on Pseudomonas Isolation Agar (PIA, Difco) plates at 37°C. Sterile cotton swabs were used to collect bacteria from the plates and to put the organisms in 2 ml 10% skim milk (Difco). Cryovials (2.5 ml, Nalgene) containing the organisms in 10% skim milk solution were subsequently stored at -80°C. All strains frozen at -80°C from the Nickerson lab were cultured on LB agar plates at 37°C and transferred to an 85% LB/15% glycerol stock solution.
2.2 E. coli strain storage
The Schurr Lab stored Escherichia coli strains in the -80°C freezer after growth in Luria-Bertani (LB) broth to an OD600 nm of 0.375. The broth culture (1 ml) was mixed with 1 ml of 65% glycerol stock solution (containing 0.1 M MgSO4 and 0.025 M Tris-HCl, pH 8.0) and stored at -80°C (F.M. Ausubel, 1993, Baker, 1998). All strains frozen at -80°C from the Nickerson lab were cultured on LB agar plates at 37°C and transferred to an 85% LB/15% glycerol stock solution.
2.3 Strain Recovery
For recovery, all strains were taken from room temperature freezers (∼30°C) at the Tulane University Health Sciences Center in New Orleans, transported to Texas (Houston for Nickerson lab and Dallas for Schurr lab), and 1 ml of each suspension was streaked on the LB agar plates with appropriate antibiotics, if necessary. Recovery was scored as positive if at least one colony was visible on the plates after incubation overnight at 37°C, however, most strains yielded hundreds of colonies on the recovery plate using this method.
2.4 Glycerol vs. skim milk survival comparison
Streptococcus pyogenes (SF370 (Ferretti et al., 2001) and JRS4 (Scott et al., 1986)), Campylobacter jejuni 81-176 (Black et al., 1988), and Borrelia burgdorferi 297 (Xu & Johnson, 1995) cultures were obtained from colleagues hosting the displaced Schurr laboratory at the University of Texas Southwestern Medical Center. Salmonella enterica subsp. Typhimurium cultures [χ3339 (Gulig & Curtiss, 1987), JW322 (Honer zu Bentrup et al., 2006), JW67 (Wilson & Nickerson, 2006)] were obtained from the Nickerson laboratory and P. aeruginosa [PAO1 (Holloway, 1955) and PAO568 (Fyfe & Govan, 1980)] and E. coli (DH-5α and DH-5α λPIR) strains from the Schurr laboratory were tested. P. aeruginosa, E. coli, and Salmonella enterica serovar Typhimurium, S. pyogenes and C. jejuni strains were grown and frozen in 10% skim milk or 15% glycerol (see Figure 1 legend). All stock suspensions were frozen at -80°C for three days and then incubated at 30°C for nine weeks, or until the glycerol stock strains were no longer viable. Serial dilutions were performed and CFUs counted every 7 days for P. aeruginosa, E. coli and S. enterica serovar Typhimurium, and every 24 hours for S. pyogenes and C. jejuni. Borrelia burgdorferi strain 297 (Xu & Johnson, 1995) (50 μl of a 1.5 ×107 Borrelia/ml culture) was frozen at -80°C in 100 μl aliquots of either 5% Difco skim milk suspensions (1:1 Borrelia culture : 10% milk solution) or 30% glycerol (final concentration) in BSK-II growth medium (Pollack et al., 1993). Two freezer vials of milk-preserved and two vials of glycerol-preserved Borrelia were placed at 30°C for 1 hr, 17 hrs, 24 hrs and 48 hrs. At the end of the incubation period, the four cultures (two milk-preserved and two glycerol-preserved) were each resuspended in 1 ml of BSK-II medium and the Borrelia allowed to recover at 34°C. Dark field examination of each culture was performed at set time points to assess spirochete survival and 10 microscopic fields per culture (at 400X magnification) were counted for bacterial enumeration.
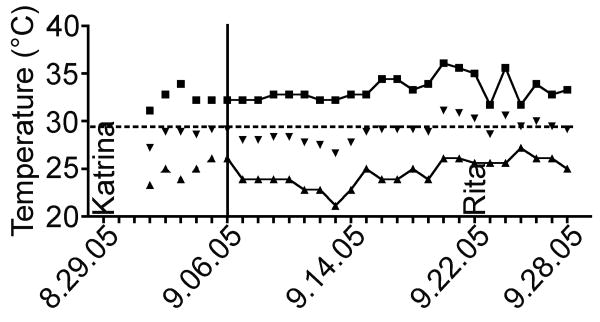
Figure 1.
New Orleans Temperatures from August 29, 2005 to September 28, 2005. High temperatures, boxes; median temperature, inverted triangles; low temperatures, triangles. Temperatures before September 6, 2005 are unofficial and demarcated by a vertical line. These were the temperatures that the Nickerson and Schurr laboratory strains endured before recovery from Tulane University Health Science Center on September 28, 2005.
3. Results and Discussion
Due to power failure, flooding and inability to enter New Orleans as a result of hurricanes Katrina and Rita, bacterial strains from the Schurr and Nickerson laboratories (as well as many other investigators in New Orleans) were kept at ambient temperature for 31 days in non-functional -80°C freezers. The exact ambient temperature of our laboratories was unknown, however reasonable approximations can be gathered from the National Climatic Data Center (http://www.ncdc.noaa.gov/oa/ncdc.html). Temperatures for Aug 29 to Sept 1 are unavailable and official measured temperatures are only available after Sept 5th. According to this data the mean outside temperature in New Orleans was 28.8 °C (Fig. 1). This temperature (∼30°C) was used to examine bacterial viability in thawed glycerol and skim milk cryopreservation solutions over time (see below).
The bacterial species recovered from the Schurr and Nickerson laboratories are listed in Table 1. The Schurr lab recovered 236 out of 258 (91.5%) of the P. aeruginosa strains stored in 10% skim milk, while only 92 out of 163 (56.4%) of E. coli strains stored in glycerol survived. The Nickerson lab, which uses 85% LB/15% glycerol for storage medium of all bacterial stocks, observed a 60% (6/10) survival rate of P. aeruginosa strains while recovering 94% of E. coli strains that were frozen. The Nickerson lab observed an 87.1% survival rate of Salmonella enterica Serovars Typhimurium and Typhi, and Salmonella bongori, collectively; a 40% survival rate of Pseudomonas putida stocks; and a 100% survival rate of Agrobacterium tumefaciens, Novosphingobium capsulatum, Rhodobacter sphaeroides and Klebsiella pneumoniae (Table 1).
Table 1.
Survival of bacterial strains after 31 days at sustained elevated temperatures of ∼30°C.
| Bacterial Species | # of Strains Examined | # of Strains Recovered | Percent Survival |
|---|---|---|---|
| Schurr Laboratory | |||
|
| |||
| Pseudomonas aeruginosa | 258 | 236 | 91.5% |
| Escherichia coli | 163 | 92 | 56.4% |
|
| |||
| Nickerson Laboratory | |||
|
| |||
| Pseudomonas aeruginosa | 10 | 6 | 60% |
| Escherichia coli sp. strain | 100 | 94 | 94% |
| TOP10 | 52 | 49 | 94.2% |
| DH5a | 15 | 14 | 93.3% |
| MG1655 | 12 | 12 | 100% |
| Other | 22 | 19 | 86.4% |
| Salmonella enterica Serovars: | 62 | 54 | 87.1% |
| Typhimurium | 56 | 48 | 85.7% |
| Typhi | 2 | 2 | 100% |
| Salmonella bongori | 4 | 4 | 100% |
| Pseudomonas putida | 5 | 2 | 40% |
| Agrobacterium tumefaciens | 7 | 7 | 100% |
| Novosphingobium capsulatum | 3 | 3 | 100% |
| Rhodobacter sphaeroides | 1 | 1 | 100% |
| Klebsiella pneumoniae | 1 | 1 | 100% |
There were differing recovery results for E. coli strains between the Nickerson (94%) and Schurr (56.4%) laboratories despite similar methods of cryopreservation (85% LB/15% glycerol vs. 50% LB/32.5% glycerol) for these strains. The lower E. coli survival rate observed by the Schurr laboratory may have been a result of the age of these strains as they had been stored at -80°C for 6 or more years. Alternately, the decreased percentage of LB in E. coli stocks from the Schurr laboratory may have affected survival after thawing. However for Pseudomonas strains, the Nickerson laboratory recovered 60% of their stocks (stored in glycerol) and the Schurr lab recovered 91.5% (stored in skim milk). Additionally, one of the Schurr laboratory personnel stored all of their bacterial stocks in 85%LB/15%glycerol and recovered 53% of their strains while another stored all of their bacterial strains in skim milk and recovered 98% (data not shown). These observations led to experiments to test if 10% skim milk or 15% glycerol was the better reagent for bacterial survival after thawing.
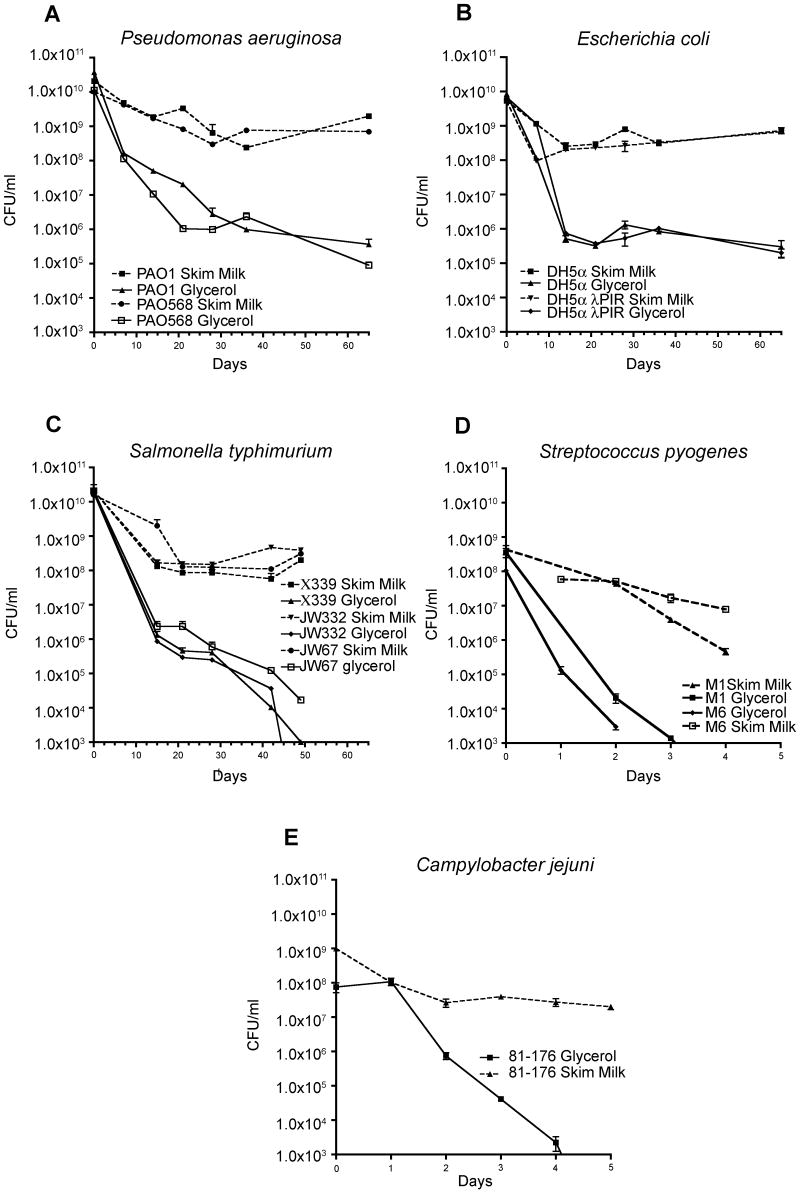
To determine if 10% skim milk or 15% glycerol was the better reagent for viability after thawing, various bacteria were collected. After 49 days incubation at 30°C, P. aeruginosa strains PAO1 and PAO568 stored in skim milk had a CFU/ml greater than 1.0×109 compared to 1×106 in glycerol stocks, demonstrating a 3-log difference in viable cells (Figure 2A). For the same period, E. coli strains DH5α and DH5α λPIR stored in skim milk contained 3-logs more viable cells than their glycerol counterparts (Figure 2B). Furthermore, all Salmonella strains stored for the same time period in glycerol were no longer viable while skim milk stored strains all produced greater than 1.0×108 CFU/ml (Figure 2C). Glycerol stored strains of S. pyogenes and C. jejuni both contained non-viable bacteria after 5 days at 30°C. Skim milk storage of these same strains produced CFUs greater than 1.0×106 and 1.0×107 respectively, at the same time point (Figure 2D & 2E). The Borrelia cultures that were frozen in the 5% skim milk solution were viable at all time points, even after incubation for 48 h at 40°C. The Borrelia cultures preserved in 30% glycerol did not survive after 1 h at 30°C (data not shown).
Figure 2.
Thawed bacterial strains survive longer in 10% skim milk than in 15% glycerol. P. aeruginosa PAO1 and PAO568 (A), E. coli DH-5α and DH-5α λPIR (B), and Salmonella enterica subsp. Typhimurium (C) were cultured on LB agar plates at 37°C overnight. S. pyogenes (D) strains were cultured on Todd-Hewitt supplemented with 2% yeast extract (THY) plates. Bacteria were collected from the plates with sterile cotton swabs and used to put the bacteria in 2 ml of 10% skim milk solution (dashed line) or a 15% glycerol solution (solid lines) in triplicate. C. jejuni 81-176 (E) was cultured on Mueller-Hinton agar containing 10 μg/ml trimethoprim. The plates were incubated for 48 h under microaerobic conditions (10% CO2, 5% O2, and 85% N2) at 37°C. Growth from each plate was collected by sterile cotton swabs and resuspended in 2.0 ml of 10% skim milk or 85% Mueller-Hinton broth and 15% glycerol. All stock suspensions were frozen at -80°C for three days and then incubated at 30°C for nine weeks, or until the glycerol stock strains were no longer viable. Serial dilutions were performed and CFUs counted every 7 days for P. aeruginosa, E. coli and S. enterica serovar Typhimurium and every 24 hours for S. pyogenes and C. jejuni.
Since it appeared that P. aeruginosa survived 49 days at relatively high CFUs (Figure 2A), we have attempted to recover 1,088 clinical P. aeruginosa Cystic Fibrosis (CF) isolates that have been at room temperature for the past 203 days. Remarkably, to date, 357/364 (98%) of the Pseudomonas CF clinical isolates stored in 10% skim milk have been recovered.
4. Conclusions
Freezing cultures at -80°C remains the most common method of preserving cultures over a prolonged period of time. Our data indicate that a 10% skim milk solution is a better cryoprotectant than the widely used 15% glycerol solution if a freezer fails for an extended period of time. Skim milk may be affecting the fatty acid content of the cell membrane, thereby altering membrane fluidity (Carvalho et al., 2004, Annous et al., 1999) or calcium may be contributing to the stability of cellular enzymes (Barach et al., 1976). We recommend the use of a 10% skim milk (wt./vol.) solution for storage to enhance long term viability of cells and protect against cell death during sustained periods of elevated temperatures.
Acknowledgments
This study was supported by NIH grant RO1AI50812 to M.J.S, NASA grants NAG 2-1378, NAG9-1350, and NCC 2-1362 to C.A.N., NIH grant RO1AI51332 to K.E.H., R01AI47928 to K.S.M., Distinguished Young Investigator Award from the President's Research Council of the University of Texas Southwestern Medical Center to D.R.H. We thank Dr. Duane Pierson and his lab at the NASA Johnson Space Center and the Department of Microbiology at the University of Texas Southwestern Medical Center (UTSMC) at Dallas for their invaluable help in preserving our strains, and to Dr. Michael Norgard and UTSMC for hosting the Schurr laboratory following the hurricane. In addition, we thank Dr. Margaret Park for her help during the challenging recovery effort of strains at Tulane.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- E F, Maniatis JST, editors. Molecular Cloning. Cold Springs Habor; 1982. Growth, Maintenance, and Preservation of Bacterial Strains; p. 62. [Google Scholar]
- Annous BA, Kozempel MF, Kurantz MJ. Changes in membrane fatty acid composition of Pediococcus sp. strain NRRL B-2354 in response to growth conditions and its effect on thermal resistance. Appl Environ Microbiol. 1999;65:2857–2862. doi: 10.1128/aem.65.7.2857-2862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aulet de Saab OC, de Castillo MC, de Ruiz Holgado AP, de Nader OM. A comparative study of preservation and storage of Haemophilus influenzae. Mem Inst Oswaldo Cruz. 2001;96:583–586. doi: 10.1590/s0074-02762001000400022. [DOI] [PubMed] [Google Scholar]
- Baker K. At the Bench: A Laboratory Navigator. Cold Springs Habor Laboratory Press; 1998. [Google Scholar]
- Barach JT, Adams DM, Speck ML. Stabilization of a psychrotrophic Pseudomonas protease by calcium against thermal inactivation in milk at ultrahigh temperature. Appl Environ Microbiol. 1976;31:875–879. doi: 10.1128/aem.31.6.875-879.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaree JM, Thompson F, Smith SJ. Use of thermal stability studies to compare Bacteroides fragilis lyophilized in skim milk and polyvinylpyrrolidone solutions. Cryobiology. 1982;19:92–98. doi: 10.1016/0011-2240(82)90128-6. [DOI] [PubMed] [Google Scholar]
- Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–479. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol Prog. 2004;20:248–254. doi: 10.1021/bp034165y. [DOI] [PubMed] [Google Scholar]
- Essiain R, Flournoy DJ. Viability of staphylococci in various diluents. Infect Control. 1986;7:370–372. doi: 10.1017/s0195941700064493. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, B R, Kingston RE, Moore D, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. In: K L, Brent R, editors. Growth on Solid Media. 1993. [Google Scholar]
- Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, Lai HS, Lin SP, Qian Y, Jia HG, Najar FZ, Ren Q, Zhu H, Song L, White J, Yuan X, Clifton SW, Roe BA, McLaughlin R. Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc Natl Acad Sci U S A. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe JAM, Govan JRW. Alginate synthesis in mucoid Pseudomonas aeruginosa: a chromosomal locus involved in control. Journal of General Microbiology. 1980;119:443–450. doi: 10.1099/00221287-119-2-443. [DOI] [PubMed] [Google Scholar]
- Gruft H, Clark ME, Osterhout M. Preservation of mycobacterial cultures. Appl Microbiol. 1968;16:355–357. doi: 10.1128/am.16.2.355-357.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig PA, Curtiss R., 3rd Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Saito M, Taguchi F, Nagaki D. Storage-effects of chorioallantoic fluid and skim milk on influenza virus infectivity. Kitasato Arch Exp Med. 1967;40:13–19. [PubMed] [Google Scholar]
- Hollander DH, Nell EE. Improved preservation of Treponema pallidum and other bacteria by freezing with glycerol. Appl Microbiol. 1954;2:164–170. doi: 10.1128/am.2.3.164-170.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway BW. Genetic recombination in Pseudomonas aeruginosa. Journal of General Microbiology. 1955;13:572–581. doi: 10.1099/00221287-13-3-572. [DOI] [PubMed] [Google Scholar]
- Honer zu Bentrup K, Ramamurthy R, Buchanan K, Emani K, Alexander S, Nelman-Gonzalez M, Wilson JW, Richter EG, Goodwin T, Ott CM, Pierson D, Nickerson CA. 3-D organotypic models of human colonic epithelium to study the early stages of enteric salmonellosis. Microbes and Infection. 2006 doi: 10.1016/j.micinf.2006.02.020. In Press. [DOI] [PubMed] [Google Scholar]
- Howard DH. The preservation of bacteria by freezing in glycerol broth. J Bacteriol. 1956;71:625. doi: 10.1128/jb.71.5.625-625.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith SC. Factors Infulencing the survival of bacteria at temperature in the vacinity of the freesing point of water. Science. 1913;37:877–881. doi: 10.1126/science.37.962.877. [DOI] [PubMed] [Google Scholar]
- Linscott WD, Boak RA. Protective action of glycerol in the freezing of leptospirae. J Bacteriol. 1960;80:573–574. doi: 10.1128/jb.80.4.573-574.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Farnum JL, Oke MA. Protective action of glycerol in the freezing of Shigella sonnei. Nature. 1962;194:405. doi: 10.1038/194405a0. [DOI] [PubMed] [Google Scholar]
- Pollack RJ, Telford SR, 3rd, Spielman A. Standardization of medium for culturing Lyme disease spirochetes. J Clin Microbiol. 1993;31:1251–1255. doi: 10.1128/jcm.31.5.1251-1255.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Guenthner PC, Malone LM, Fischetti VA. Conversion of an M- group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J Exp Med. 1986;164:1641–1651. doi: 10.1084/jem.164.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha RN, Dudani AT, Ranganathan B. Effect of individual ingredients of fortified skim milk as suspending media on survival of freeze-dried cells of Streptococcus lactis. Cryobiology. 1974;11:368–370. doi: 10.1016/0011-2240(74)90014-5. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ito S, Yoshida K. Effects of freezing and thawing and storage by freezing on the biological and morphological properties of strain K-9 of Klebsiella pneumoniae and its variants. Z Allg Mikrobiol. 1982;22:649–660. doi: 10.1002/jobm.3630220906. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Nickerson CA. A new experimental approach for studying bacterial genomic island evolution identifies island genes with bacterial host-specific expression patterns. BMC Evol Biol. 2006;6:2. doi: 10.1186/1471-2148-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Johnson RC. Analysis and comparison of plasmid profiles of Borrelia burgdorferi sensu lato strains. J Clin Microbiol. 1995;33:2679–2685. doi: 10.1128/jcm.33.10.2679-2685.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]