Abstract
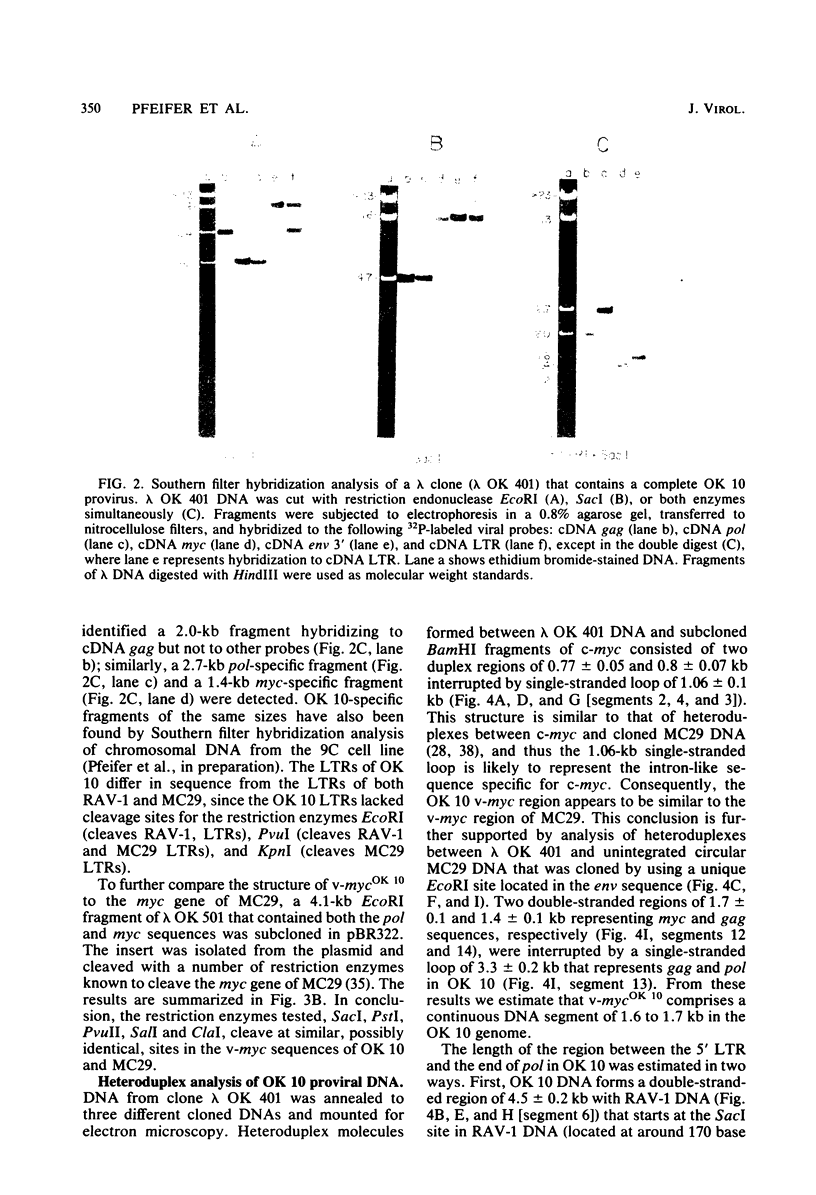
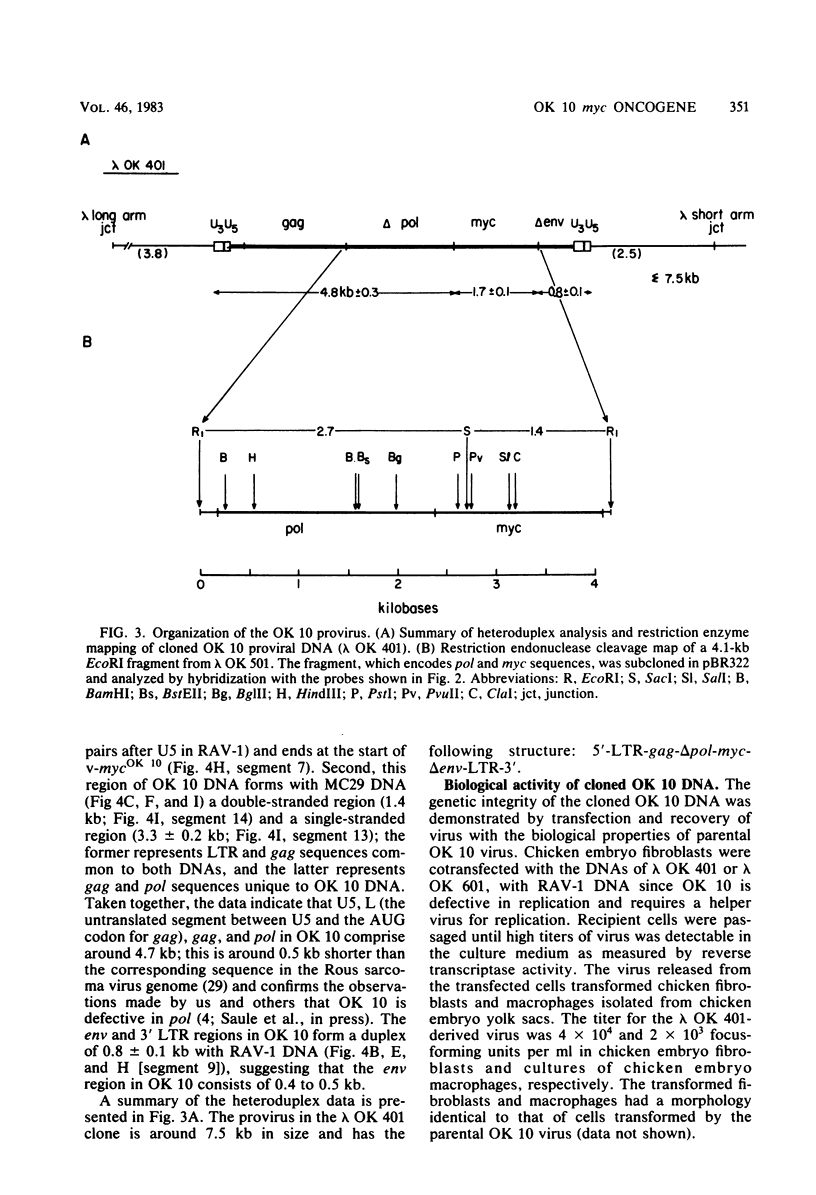
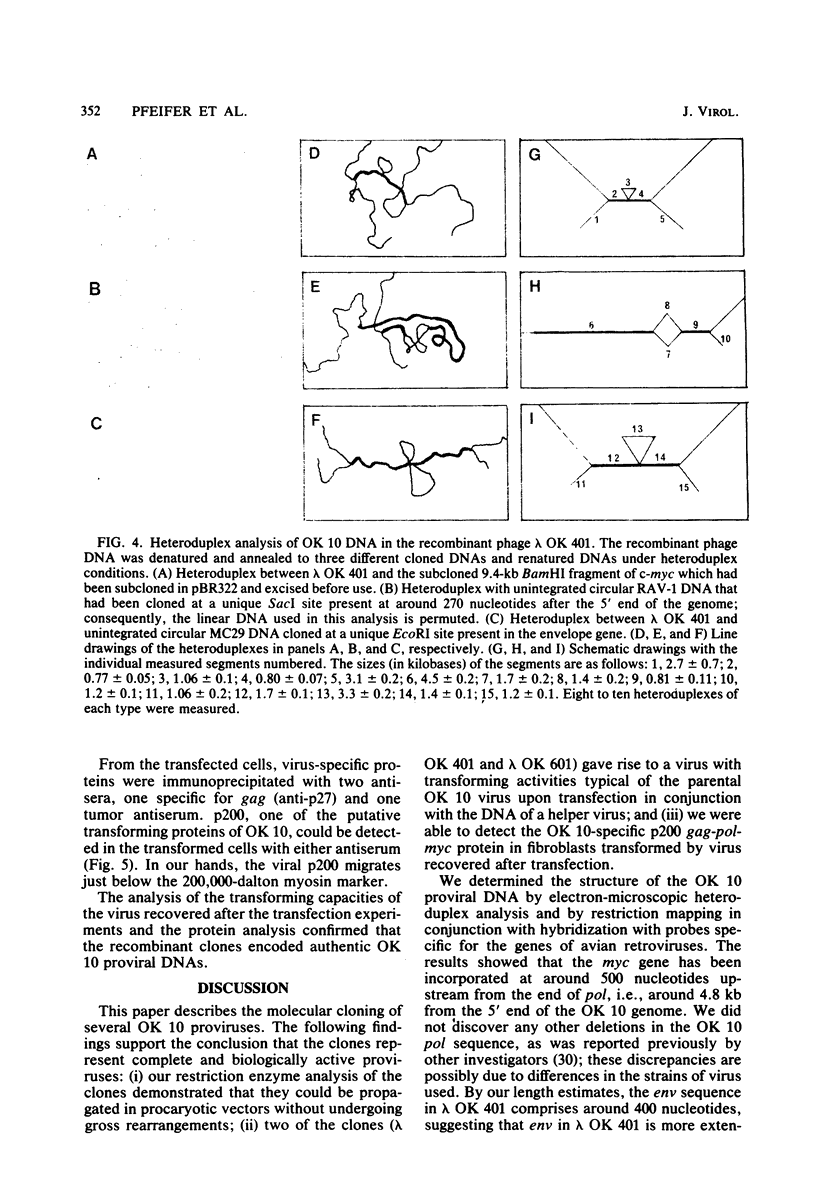
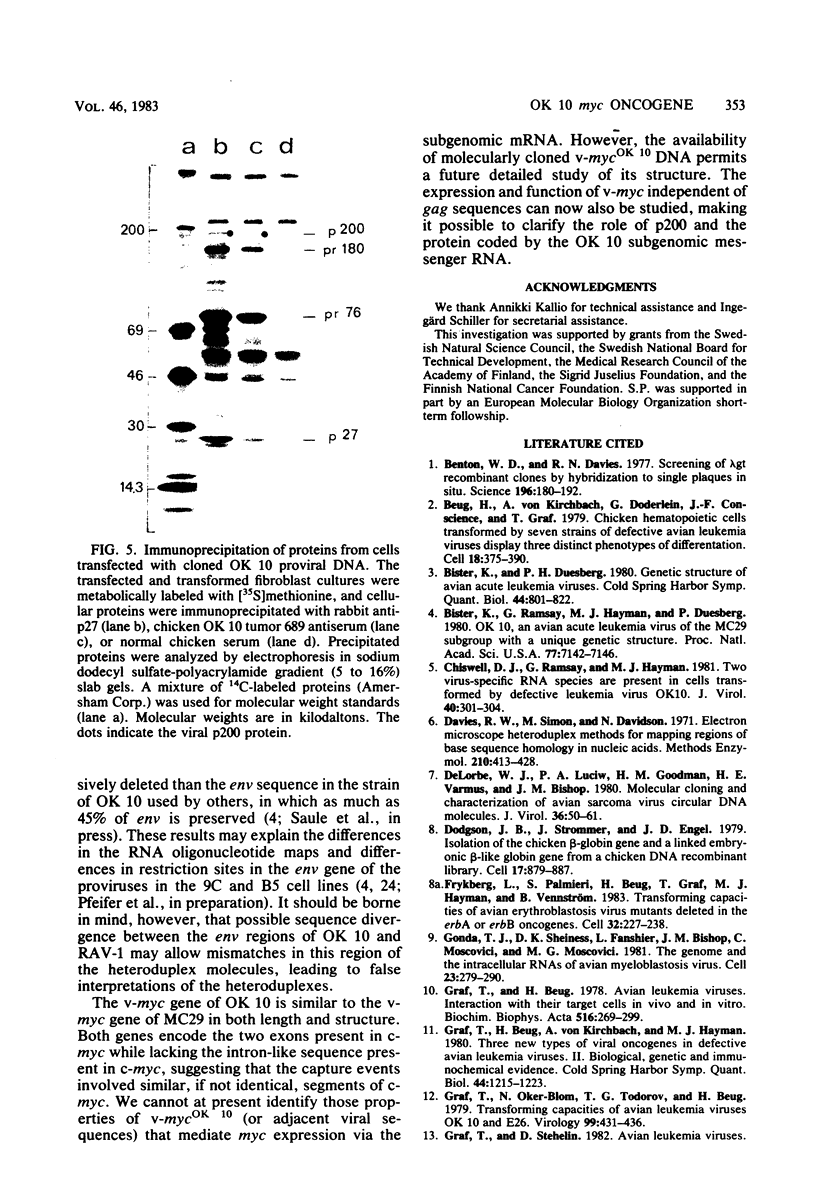
Several DNAs representing the genome of the avian acute leukemia virus OK 10 were isolated by molecular cloning from a transformed quail cell line, 9C, which contained at least six OK 10 proviruses. Recombinant lambda phages harboring the OK 10 genome and additional flanking cellular DNA sequences were studied by restriction endonuclease mapping and hybridization to viral cDNA probes. Six of the clones represented complete proviruses with similar, if not identical, viral sequences integrated at different positions in the host DNA. The organization of the OK 10 genome was determined by electron-microscopic analysis of heteroduplexes formed between the cloned OK 10 DNA and DNAs representing the c-myc gene and the genomes of two other avian retroviruses, Rous-associated virus-1 and MC29. The results indicated that the OK 10 proviral DNA is about 7.5 kilobases in size with the following structure: 5'-LTR-gag-delta polmyc-delta env-LTR-3', where LTR indicates a long terminal repeat. The oncogene of OK 10, v-mycOK 10, forms a continuous DNA segment of around 1.7 kilobases between pol and env. It is similar in structure and length to the v-myc gene of MC29, as demonstrated by restriction endonuclease and heteroduplex analyses. Two of the OK 10 proviruses were tested in transfection experiments: both DNAs gave rise to virus with the transforming capacities of OK 10 when Rous-associated virus-1 was used to provide helper virus functions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Ramsay G., Hayman M. J., Duesberg P. H. OK10, an avian acute leukemia virus of the MC 29 subgroup with a unique genetic structure. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7142–7146. doi: 10.1073/pnas.77.12.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswell D. J., Ramsay G., Hayman M. J. Two virus-specific rna species are present in cells transformed by defective leukemia virus OK10. J Virol. 1981 Oct;40(1):301–304. doi: 10.1128/jvi.40.1.301-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Fanshier L., Bishop J. M., Moscovici C., Moscovici M. G. The genome and the intracellular RNAs of avian myeloblastosis virus. Cell. 1981 Jan;23(1):279–290. doi: 10.1016/0092-8674(81)90292-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Stéhelin D. Avian leukemia viruses. Oncogenes and genome structure. Biochim Biophys Acta. 1982 Jun 28;651(4):245–271. doi: 10.1016/0304-419x(82)90014-2. [DOI] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Hortling L. Rapid oncogenesis in vivo by chicken retrovirus OK10. Acta Pathol Microbiol Scand B. 1978 Aug;86(4):185–192. [PubMed] [Google Scholar]
- Hughes S. H., Payvar F., Spector D., Schimke R. T., Robinson H. L., Payne G. S., Bishop J. M., Varmus H. E. Heterogeneity of genetic loci in chickens: analysis of endogenous viral and nonviral genes by cleavage of DNA with restriction endonucleases. Cell. 1979 Oct;18(2):347–359. doi: 10.1016/0092-8674(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lautenberger J. A., Schulz R. A., Garon C. F., Tsichlis P. N., Papas T. S. Molecular cloning of avian myelocytomatosis virus (MC29) transforming sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1518–1522. doi: 10.1073/pnas.78.3.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovici C., Gazzolo L., Moscovici M. G. Focus assay and defectiveness of avian myeloblastosis virus. Virology. 1975 Nov;68(1):173–181. doi: 10.1016/0042-6822(75)90159-2. [DOI] [PubMed] [Google Scholar]
- Oker-Blom N., Hortling L., Kallio A., Nurmiaho E. L., Westermarck H. OK 10 virus, an avian retrovirus resembling the acute leukaemia viruses. J Gen Virol. 1978 Sep;40(3):623–633. doi: 10.1099/0022-1317-40-3-623. [DOI] [PubMed] [Google Scholar]
- Pfeifer S., Kallio A., Vaheri A., Pettersson R., Oker-Blom N. Stable bone-marrow-derived cell line producing transforming avian acute leukemia virus OK 10. Int J Cancer. 1980 Feb 15;25(2):235–242. doi: 10.1002/ijc.2910250211. [DOI] [PubMed] [Google Scholar]
- Pfeifer S., Pettersson R. F., Kallio A., Oker-Blom N., Vaheri A. Avian acute leukemia virus OK10 has an 8.2-kilobase genome and modified glycoprotein gp 78. J Virol. 1981 Nov;40(2):533–540. doi: 10.1128/jvi.40.2.533-540.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J. Analysis of cells transformed by defective leukemia virus OK10: production of noninfectious particles and synthesis of Pr76gag and an additional 200,000-dalton protein. Virology. 1980 Oct 15;106(1):71–81. doi: 10.1016/0042-6822(80)90222-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins T., Bister K., Garon C., Papas T., Duesberg P. Structural relationship between a normal chicken DNA locus and the transforming gene of the avian acute leukemia virus MC29. J Virol. 1982 Feb;41(2):635–642. doi: 10.1128/jvi.41.2.635-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Bishop J. M. DNA and RNA from uninfected vertebrate cells contain nucleotide sequences related to the putative transforming gene of avian myelocytomatosis virus. J Virol. 1979 Aug;31(2):514–521. doi: 10.1128/jvi.31.2.514-521.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Vennstrom B., Bishop J. M. Virus-specific RNAs in cells infected by avian myelocytomatosis virus and avian erythroblastosis virus: modes of oncogene expression. Cell. 1981 Jan;23(1):291–300. doi: 10.1016/0092-8674(81)90293-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Saule S., Roussel M., Sergeant A., Lagrou C., Rommens C., Raes M. B. Three new types of viral oncogenes in defective avian leukemia viruses. I. Specific nucleotide sequences of cellular origin correlate with specific transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1215–1223. doi: 10.1101/sqb.1980.044.01.132. [DOI] [PubMed] [Google Scholar]
- Vennstrom B., Sheiness D., Zabielski J., Bishop J. M. Isolation and characterization of c-myc, a cellular homolog of the oncogene (v-myc) of avian myelocytomatosis virus strain 29. J Virol. 1982 Jun;42(3):773–779. doi: 10.1128/jvi.42.3.773-779.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]