Abstract
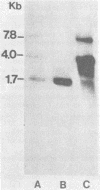
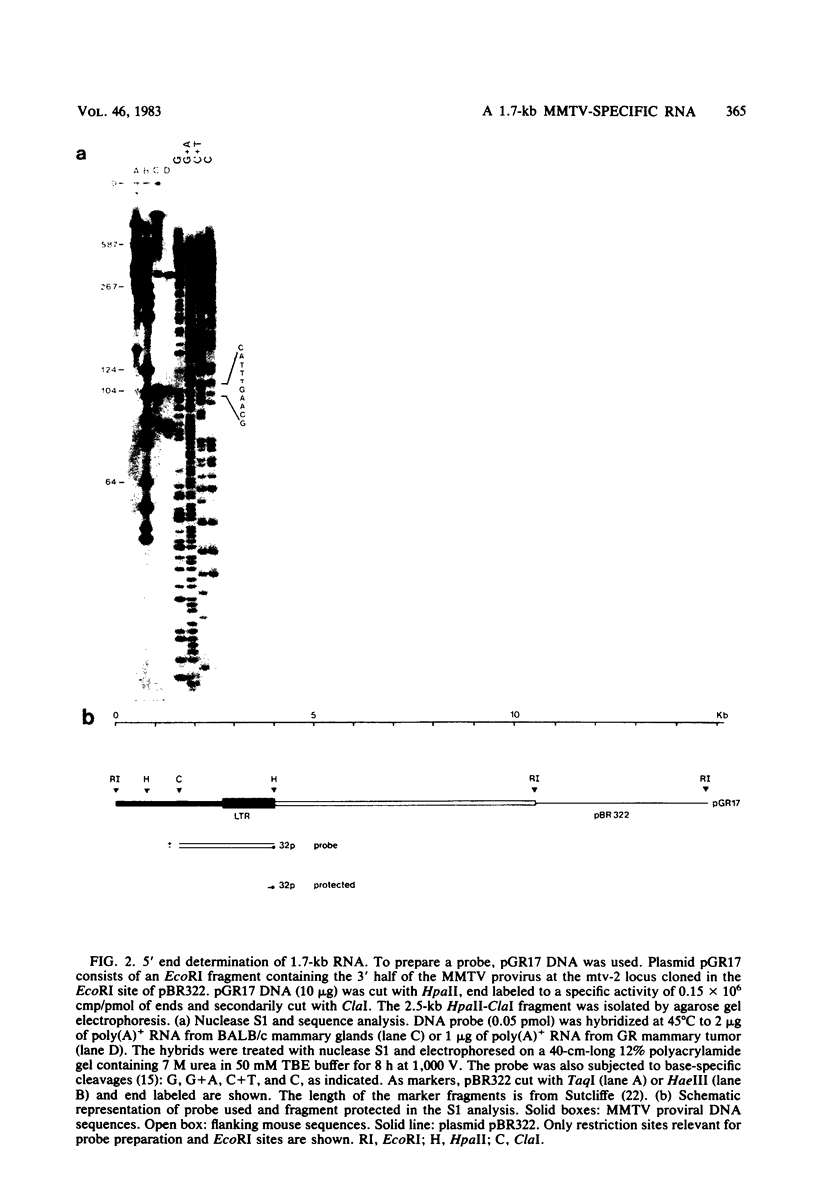
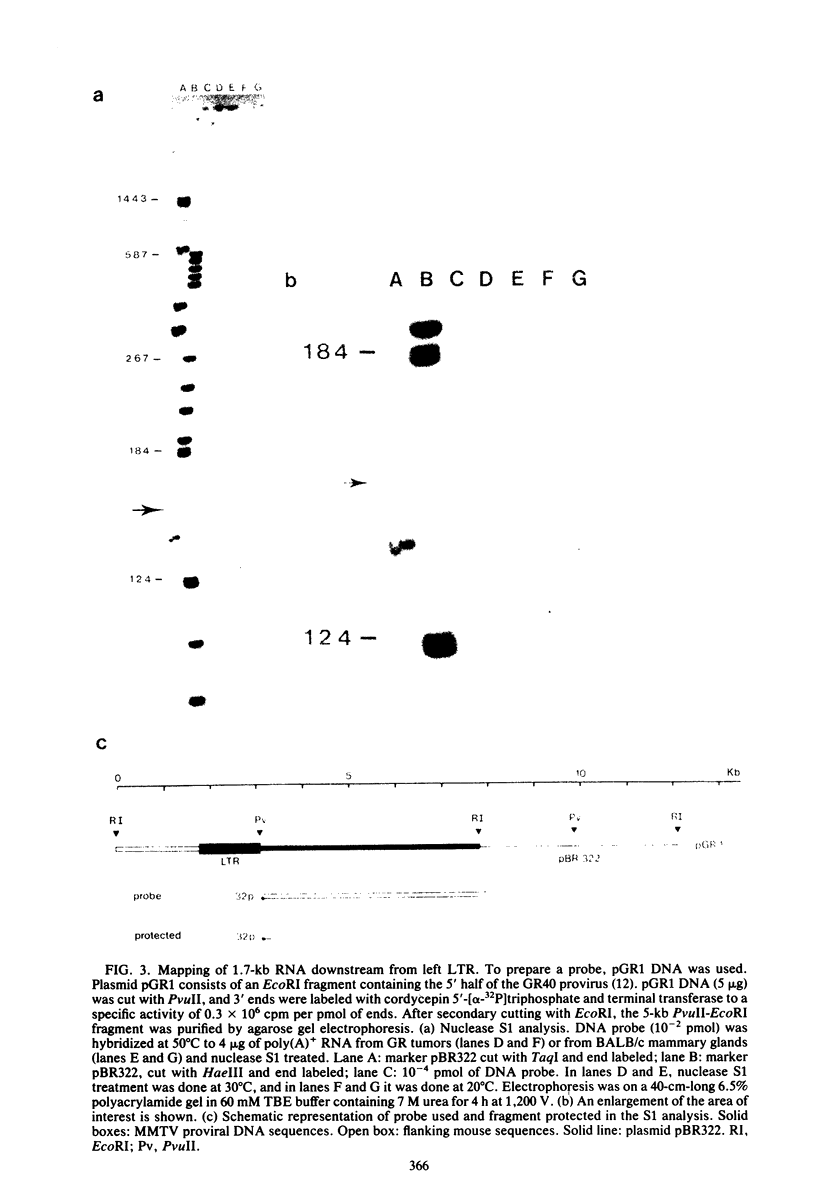
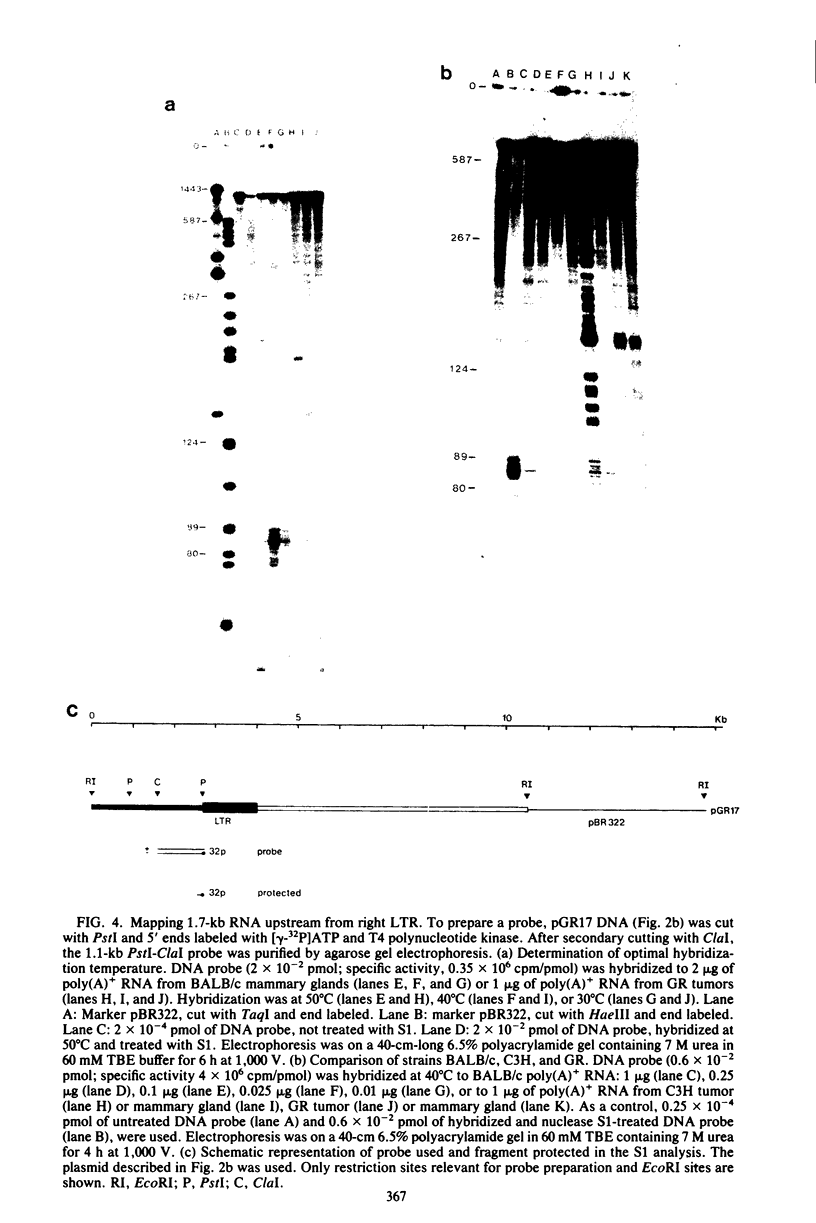
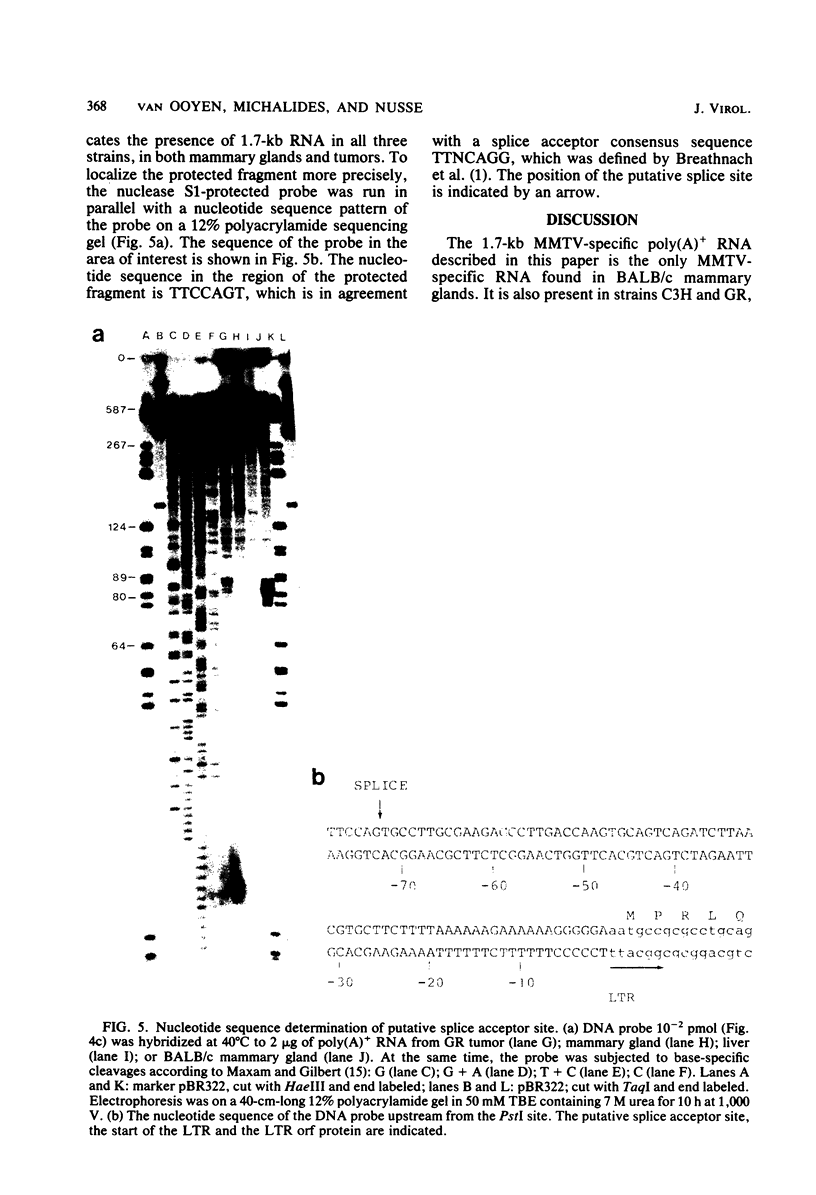
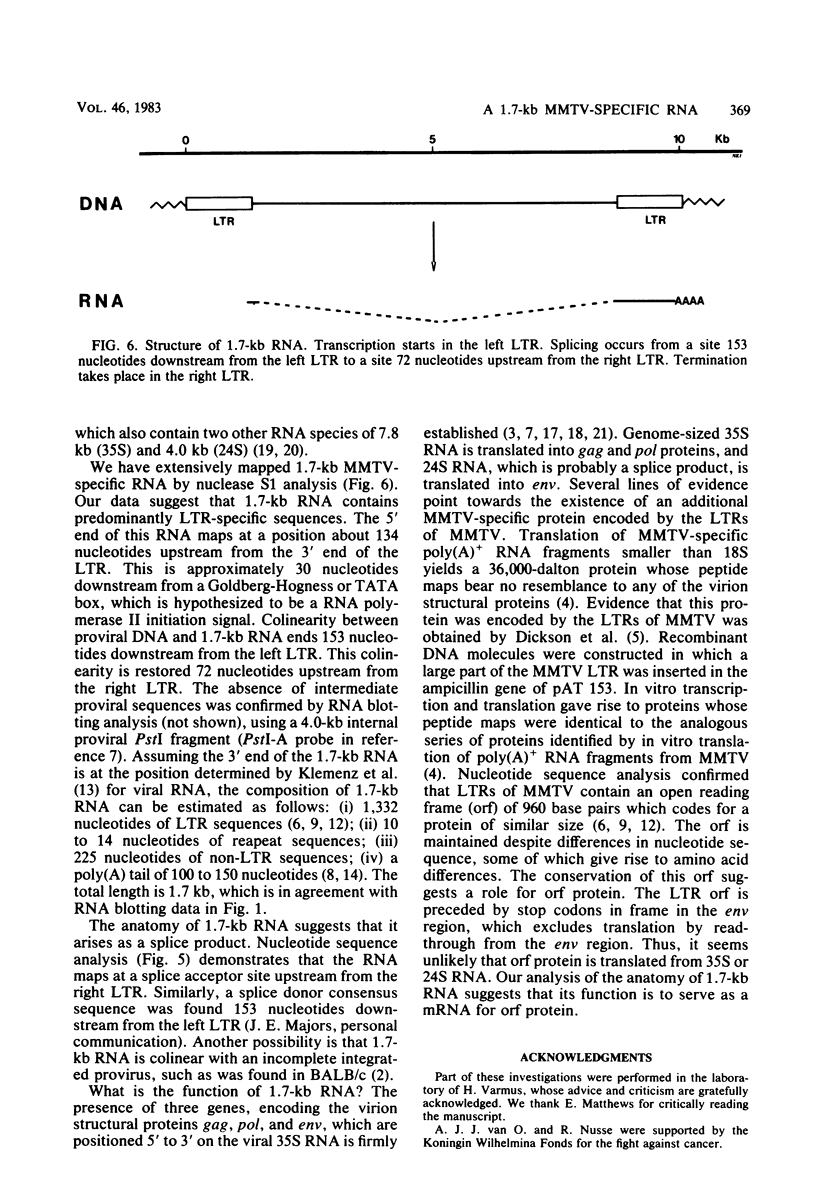
We have detected a mouse mammary tumor virus (MMTV)-specific 1.7-kilobase (kb) polyadenylated RNA in mammary glands of several mouse strains. In BALB/c mice, it is the only MMTV-specific RNA species present. C3H and GR mammary glands and tumors contain, in addition, 3.8- and 7.8-kb MMTV RNAs. Nuclease S1 analysis was performed to map 1.7-kb polyadenylated RNA. It contains predominantly long terminal repeat (LTR) sequences. The 5' end maps approximately 134 nucleotides upstream from the 3' end of the LTR. Colinearity with complete proviral DNA continues to a site about 153 nucleotides downstream from the left (5') LTR. No sequences from the middle part of proviral DNA were found. Colinearity with proviral DNA is resumed 72 nucleotides upstream from the right (3') LTR. The nucleotide sequence in this area is TTCCAGT, which is a splice acceptor consensus sequence. The anatomy of 1.7-kb RNA indicates that it may serve as a messenger for the 36,700-dalton protein encoded by the LTRs of MMTV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Smith R., Peters G. In vitro synthesis of polypeptides encoded by the long terminal repeat region of mouse mammary tumour virus DNA. Nature. 1981 Jun 11;291(5815):511–513. doi: 10.1038/291511a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P., Varmus H. E. Purification and translation of murine mammary tumor virus mRNA's. J Virol. 1981 Jul;39(1):207–218. doi: 10.1128/jvi.39.1.207-218.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds M., Caramela M. G. The isolation and characterization of adenosine monophosphate-rich polynucleotides synthesized by Ehrlich ascites cells. J Biol Chem. 1969 Mar 10;244(5):1314–1324. [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E. Number and location of mouse mammary tumor virus proviral DNA in mouse DNA of normal tissue and of mammary tumors. J Virol. 1980 Mar;33(3):1013–1025. doi: 10.1128/jvi.33.3.1013-1025.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes N. E., Kennedy N., Rahmsdorf U., Groner B. Hormone-responsive expression of an endogenous proviral gene of mouse mammary tumor virus after molecular cloning and gene transfer into cultured cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2038–2042. doi: 10.1073/pnas.78.4.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy N., Knedlitschek G., Groner B., Hynes N. E., Herrlich P., Michalides R., van Ooyen A. J. Long terminal repeats of endogenous mouse mammary tumour virus contain a long open reading frame which extends into adjacent sequences. Nature. 1982 Feb 18;295(5850):622–624. doi: 10.1038/295622a0. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Reinhardt M., Diggelmann H. Sequence determination of the 3' end of mouse mammary tumor virus RNA. Mol Biol Rep. 1981 May 22;7(1-3):123–126. doi: 10.1007/BF00778742. [DOI] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Nie R., Nusse R., Hynes N. E., Groner B. Mammary tumor induction loci in GR and DBAf mice contain one provirus of the mouse mammary tumor virus. Cell. 1981 Jan;23(1):165–173. doi: 10.1016/0092-8674(81)90281-6. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Nusse R., de Moes J., Hilkens J., van Nie R. Localization of a gene for expression of mouse mammary tumor virus antigens in the GR/Mtv-2- mouse strain. J Exp Med. 1980 Sep 1;152(3):712–719. doi: 10.1084/jem.152.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Dexamethasone induction of the intracellular RNAs of mouse mammary tumor virus. J Virol. 1981 Dec;40(3):673–682. doi: 10.1128/jvi.40.3.673-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]