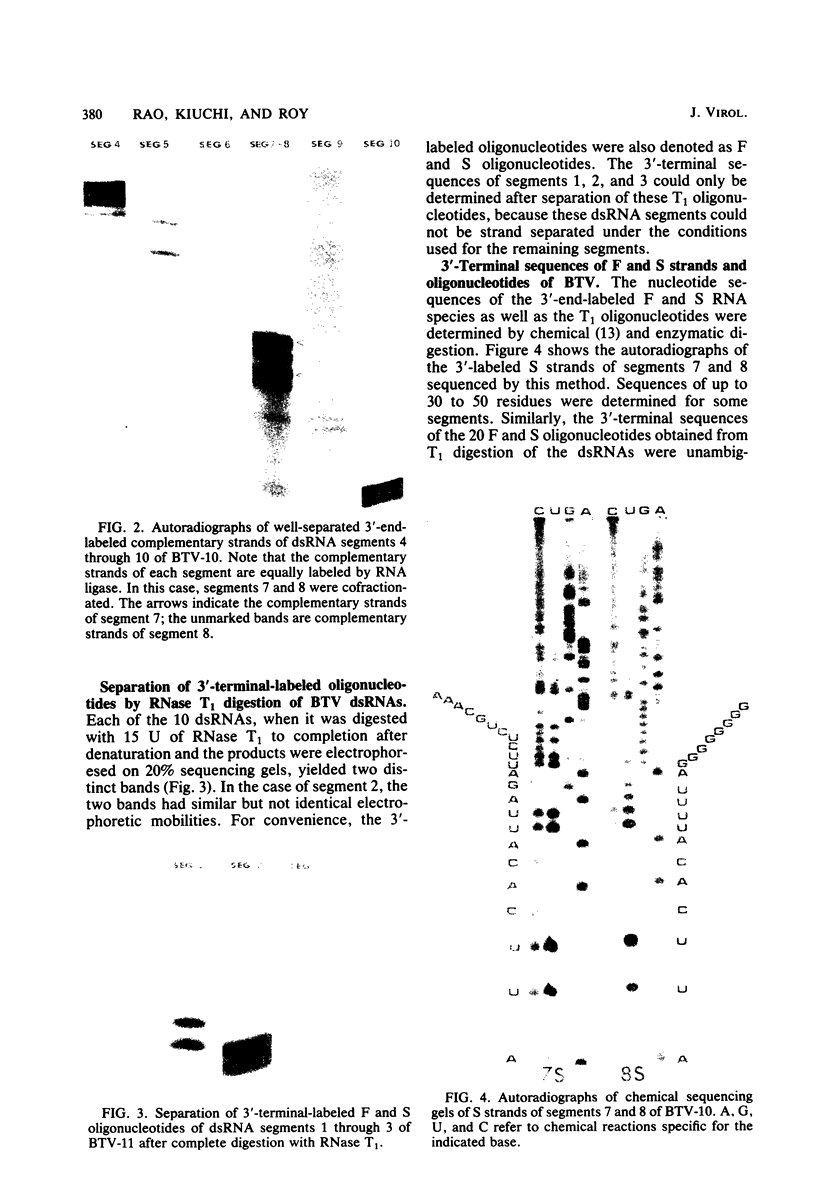
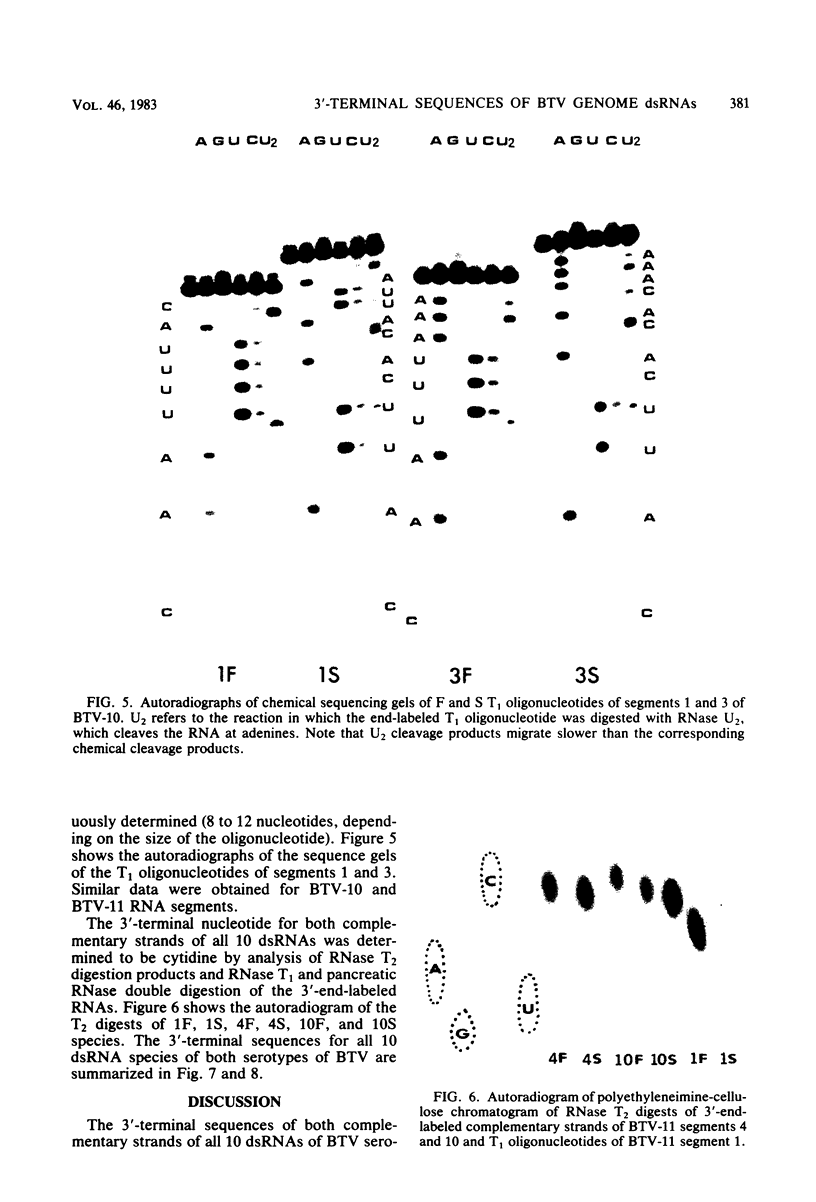
Abstract
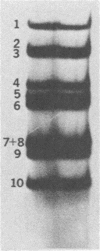
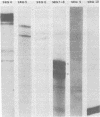
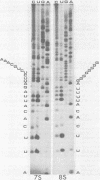
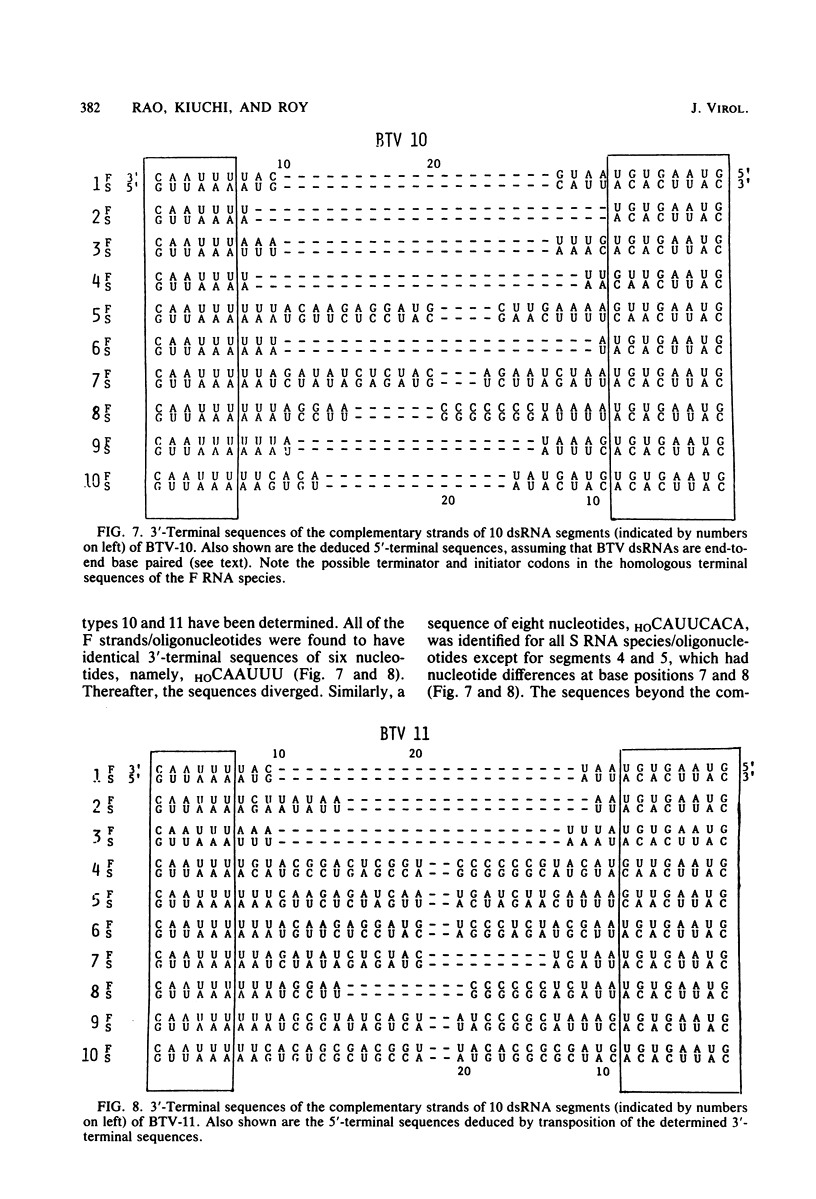
The 3'-terminal sequences of the 10 double-stranded RNA genome segments of bluetongue virus (serotypes 10 and 11) were determined. The double-stranded RNAs were 3' labeled with [5'-32P]pCp and resolved into 10 segments by electrophoresis. After denaturation, the two complementary strands of segments 4 through 10 were resolved into fast- and slow-migrating species by polyacrylamide gel electrophoresis, and their 3' end sequences were determined. Complete RNase T1 digestion of the individual 3'-labeled double-stranded RNA segments yielded two labeled oligonucleotides, one of which migrated faster than the other on 20% polyacrylamide-7 M urea gels. Sequence analyses of the two oligonucleotides of segments 4 through 10 confirmed the corresponding RNA sequence data. For RNA segments 1 through 3 the oligonucleotide analyses gave comparable results. The 3'-terminal sequences of the fast-migrating RNA species were HOCAAUUU. . . ; those of the slow-migrating RNA species were HOCAUUCACA. . . . Similar results were obtained for double-stranded RNA from bluetongue virus serotypes 10 and 11. Beyond the common termini, the sequences for each segment varied considerably.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Darzynkiewicz E., Shatkin A. J. Assignment of reovirus mRNA ribosome binding sites to virion genome segments by nucleotide sequence analyses. Nucleic Acids Res. 1980 Jan 25;8(2):337–350. doi: 10.1093/nar/8.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz J. R., Kaper J. M. Isolation of viral double-stranded RNAs using a LiCl fractionation procedure. Prep Biochem. 1978;8(1):1–17. doi: 10.1080/00327487808068215. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Gorman B. M. Variation in orbiviruses. J Gen Virol. 1979 Jul;44(1):1–15. doi: 10.1099/0022-1317-44-1-1. [DOI] [PubMed] [Google Scholar]
- Hastings K. E., Millward S. Nucleotide sequences at the 5' termini of reovirus mRNA's. J Virol. 1978 Nov;28(2):490–498. doi: 10.1128/jvi.28.2.490-498.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences and properties of two ribosome binding sites from the small size class of reovirus messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6895–6908. [PubMed] [Google Scholar]
- Kozak M., Shatkin A. J. Sequences of two 5'-terminal ribosome-protected fragments from reovirus messenger RNAs. J Mol Biol. 1977 May 5;112(1):75–96. doi: 10.1016/s0022-2836(77)80157-5. [DOI] [PubMed] [Google Scholar]
- Li J. K., Keene J. D., Scheible P. P., Joklik W. K. Nature of the 3'-terminal sequences of the plus and minus strands of the S1 gene of reovirus serotypes 1, 2 and 3. Virology. 1980 Aug;105(1):41–51. doi: 10.1016/0042-6822(80)90154-3. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Zweerink H. J. Isolation and characterization of two types of bluetongue virus particles. Virology. 1972 Nov;50(2):495–506. doi: 10.1016/0042-6822(72)90400-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCrae M. A. Terminal structure of reovirus RNAs. J Gen Virol. 1981 Aug;55(Pt 2):393–403. doi: 10.1099/0022-1317-55-2-393. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. A micromethod for base analysis of 32P-labeled oligoribonulcleotides. Anal Biochem. 1977 Nov;83(1):222–227. doi: 10.1016/0003-2697(77)90530-9. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Fiers W. Micro thin-layer techniques for rapid sequence analysis of 32P-labeled RNA: double digestion and pancreatic ribonuclease analyses. Anal Biochem. 1977 Nov;83(1):228–239. doi: 10.1016/0003-2697(77)90531-0. [DOI] [PubMed] [Google Scholar]
- Wieslander L. A simple method to recover intact high molecular weight RNA and DNA after electrophoretic separation in low gelling temperature agarose gels. Anal Biochem. 1979 Oct 1;98(2):305–309. doi: 10.1016/0003-2697(79)90145-3. [DOI] [PubMed] [Google Scholar]