Abstract
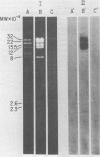
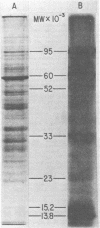
Purified virions of milker's nodule virus, a parapoxvirus, were shown to contain an RNA polymerase, a nucleotide phosphohydrolase, and a protein kinase associated with or encapsulated within the DNA-containing core of the virus. In vitro, the activated viral RNA polymerase transcribed only 7 to 8% of the genome, in the form of 8S to 14S polyadenylated RNA molecules which were complementary to sequences present in milker's nodule virus DNA but not vaccinia virus DNA or DNA prepared from the host cells in which the virus was propagated. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis showed that in vitro, the activated viral protein kinase phosphorylated viral polypeptides of 95, 60, 33.5, 15, and 13.8 kilodaltons.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arzoglou P., Drillien R., Kirn A. Evidence for an alkaline protease in vaccinia virus. Virology. 1979 May;95(1):211–214. doi: 10.1016/0042-6822(79)90416-1. [DOI] [PubMed] [Google Scholar]
- Aubertin A. M., McAuslan B. R. Virus-associated nucleases: evidence for endonuclease and exonuclease activity in rabbitpox and vaccinia viruses. J Virol. 1972 Mar;9(3):554–556. doi: 10.1128/jvi.9.3.554-556.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER Y., JOKLIK W. K. MESSENGER RNA IN CELLS INFECTED WITH VACCINIA VIRUS. Proc Natl Acad Sci U S A. 1964 Apr;51:577–585. doi: 10.1073/pnas.51.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUETTNER D., GIESE H., MUELLER G., PETERS D. DIE FEINSTRUKTUR REIFER ELEMENTARKOERPER DES ECTHYMA CONTAGIOSUM UND DER STOMATITIS PAPULOSA. Arch Gesamte Virusforsch. 1964 Jun 17;14:657–673. [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Bauer W. R., Ressner E. C., Kates J., Patzke J. V. A DNA nicking-closing enzyme encapsidated in vaccinia virus: partial purification and properties. Proc Natl Acad Sci U S A. 1977 May;74(5):1841–1845. doi: 10.1073/pnas.74.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Silverman C. Natural occurrence of cross-linked vaccinia virus deoxyribonucleic acid. J Virol. 1970 Mar;5(3):299–304. doi: 10.1128/jvi.5.3.299-304.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn P. Ribonuclease inhibitor from human placenta: rapid purification and assay. J Biol Chem. 1979 Dec 25;254(24):12484–12487. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Dorson J. W., Bollum F. J. Terminal riboadenylate transferase: a poly A polymerase in purified vaccinia virus. J Virol. 1973 Aug;12(2):203–208. doi: 10.1128/jvi.12.2.203-208.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN E. P., DELONG S. S., SANDERS J., MOSCOVICI C. ASPECTS OF THE MORPHOLOGIC DEVELOPMENT OF PSEUDOCOWPOX VIRUS. Virology. 1964 May;23:56–64. doi: 10.1016/s0042-6822(64)80007-6. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Esteban M., McCarron R., McAllister W. T., Holowczak J. A. Vaccinia virus transcription: hybridization of mRNA to restriction fragments of vaccinia DNA. Virology. 1978 May 1;86(1):102–114. doi: 10.1016/0042-6822(78)90011-9. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Downer D. N., Rogers H. W., Randall C. C. Endogenous protein kinase and phosphate acceptor proteins in vaccinia virus. Virology. 1973 Mar;52(1):13–21. doi: 10.1016/0042-6822(73)90393-0. [DOI] [PubMed] [Google Scholar]
- Easterbrook K. B. Controlled degradation of vaccinia virions in vitro: an electron microscopic study. J Ultrastruct Res. 1966 Mar;14(5):484–496. doi: 10.1016/s0022-5320(66)80077-1. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Martin S. A., Paoletti E., Moss B. Modification of the 5'-terminus of mRNA by soluble guanylyl and methyl transferases from vaccinia virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2525–2529. doi: 10.1073/pnas.72.7.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F. The biological characters of several strains of vaccinia, cowpox and rabbitpox viruses. Virology. 1958 Jun;5(3):502–529. doi: 10.1016/0042-6822(58)90042-4. [DOI] [PubMed] [Google Scholar]
- FRIEDMAN-KIEN A. E., ROWE W. P., BANFIELD W. G. Milker's nodules: isolation of a poxvirus from a human case. Science. 1963 Jun 21;140(3573):1335–1336. doi: 10.1126/science.140.3573.1335. [DOI] [PubMed] [Google Scholar]
- Fenner F. Portraits of viruses: the poxviruses. Intervirology. 1979;11(3):137–157. doi: 10.1159/000149027. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. M., Parsonage M. T., Pelham H. R., Darby G. Heat inactivation of vaccinia virus particle-associated functions: properties of heated particles in vivo and in vitro. J Virol. 1978 Jun;26(3):646–659. doi: 10.1128/jvi.26.3.646-659.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Condit R. C., Kates J. R. Cellular differences in the molecular mechanisms of vaccinia virus host range restriction. J Gen Virol. 1980 Apr;47(2):485–488. doi: 10.1099/0022-1317-47-2-485. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. The poxviruses. Bacteriol Rev. 1966 Mar;30(1):33–66. doi: 10.1128/br.30.1.33-66.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. I. The mechanism of synthesis and release of RNA in vaccinia cores. J Mol Biol. 1970 May 28;50(1):1–18. doi: 10.1016/0022-2836(70)90100-2. [DOI] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOSCOVICI C., COHEN E. P., SANDERS J., DELONG S. S. ISOLATION OF A VIRAL AGENT FROM PSEUDOCOWPOX DISEASE. Science. 1963 Sep 6;141(3584):915–916. doi: 10.1126/science.141.3584.915. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Moss B. Modification of RNA by mRNA guanylyltransferase and mRNA (guanine-7-)methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9330–9335. [PubMed] [Google Scholar]
- Martin S. A., Moss B. mRNA guanylyltransferase and mRNA (guanine-7-)-methyltransferase from vaccinia virions. Donor and acceptor substrate specificites. J Biol Chem. 1976 Dec 10;251(23):7313–7321. [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- McCarron R. J., Cabrera C. V., Esteban M., McAllister W. T., Holowczak J. A. Structure of vaccinia DNA: analysis of the viral genome by restriction endonucleases. Virology. 1978 May 1;86(1):88–101. doi: 10.1016/0042-6822(78)90010-7. [DOI] [PubMed] [Google Scholar]
- Menna A., Wittek R., Bachmann P. A., Mayr A., Wyler R. Physical characterization of a stomatitis papulosa virus genome: a cleavage map for the restriction endonucleases HindIII and EcoRI. Arch Virol. 1979;59(1-2):145–156. doi: 10.1007/BF01317904. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Gershowitz A. Characterization of a polyriboadenylate polymerase from vaccinia virions. J Biol Chem. 1975 Jun 25;250(12):4722–4729. [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Paoletti E. Polyadenylate polymerase from vaccinia virions. Nat New Biol. 1973 Sep 12;245(141):59–63. doi: 10.1038/newbio245059a0. [DOI] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGINGTON J., HORNE R. W. Morphological studies of orf and vaccinia viruses. Virology. 1962 Mar;16:248–260. doi: 10.1016/0042-6822(62)90245-3. [DOI] [PubMed] [Google Scholar]
- NAGINGTON J., NEWTON A. A., HORNE R. W. THE STRUCTURE OF ORF VIRUS. Virology. 1964 Aug;23:461–472. doi: 10.1016/0042-6822(64)90230-2. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- PETERS D., MUELLER G., BUETTNER D. THE FINE STRUCTURE OF PARAVACCINIA VIRUSES. Virology. 1964 Aug;23:609–611. doi: 10.1016/0042-6822(64)90246-6. [DOI] [PubMed] [Google Scholar]
- Paolette E., Rosemond-Hornbeak H., Moss B. Two nucleid acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3273–3280. [PubMed] [Google Scholar]
- Paoletti E., Cooper N., Moss B. Regulation of synthesis of two immunologically distinct nucleic acid-dependent nucleoside triphosphate phosphohydrolases in vaccinia virus-infected HeLa cells. J Virol. 1974 Sep;14(3):578–586. doi: 10.1128/jvi.14.3.578-586.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Grady L. J. Transcriptional complexity of vaccinia virus in vivo and in vitro. J Virol. 1977 Sep;23(3):608–615. doi: 10.1128/jvi.23.3.608-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E. High molecular weight virion-associated RNA of vaccinia. A possible precursor to 8 to 12 S mRNA. J Biol Chem. 1977 Feb 10;252(3):872–877. [PubMed] [Google Scholar]
- Paoletti E. In vitro synthesis of a high molecular weight virion-associated RNA by vaccinia. J Biol Chem. 1977 Feb 10;252(3):866–871. [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R. Soluble endoribonuclease activity from vaccinia virus: specific cleavage of virion-associated high-molecular-weight RNA. J Virol. 1978 Jun;26(3):822–824. doi: 10.1128/jvi.26.3.822-824.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R. The role of ATP in the biogenesis of vaccinia virus mRNA in vitro. Virology. 1978 Jun 15;87(2):317–325. doi: 10.1016/0042-6822(78)90137-x. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Deoxyribonucleic acid-dependent nucleotide phosphohydrolase activity in purified vaccinia virus. J Virol. 1972 Oct;10(4):866–868. doi: 10.1128/jvi.10.4.866-868.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Two nucleic acid-dependent nucleoside triphosphate phosphohydrolases from vaccinia virus. Nucleotide substrate and polynucleotide cofactor specificities. J Biol Chem. 1974 May 25;249(10):3281–3286. [PubMed] [Google Scholar]
- Parr R. P., Burnett J. W., Garon C. F. Ultrastructural characterization of the Molluscum contagiosum virus genome. Virology. 1977 Sep;81(2):247–256. doi: 10.1016/0042-6822(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S., Bergoin M., Roberts D. W. Enzymes associated with an insect poxvirus. Virology. 1971 Jan;43(1):306–309. doi: 10.1016/0042-6822(71)90249-2. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Dales S. Two deoxyribonuclease activities within purified vaccinia virus. Proc Natl Acad Sci U S A. 1969 Jul;63(3):820–827. doi: 10.1073/pnas.63.3.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospischil A., Bachmann P. A. Nuclear changes in cells infected with parapoxviruses stomatitis papulosa and orf: an in vivo and in vitro ultrastructural study. J Gen Virol. 1980 Mar;47(1):113–121. doi: 10.1099/0022-1317-47-1-113. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Paoletti E., Moss B. Single-stranded deoxyribonucleic acid-specific nuclease from vaccinia virus. Purification and characterization. J Biol Chem. 1974 May 25;249(10):3287–3291. [PubMed] [Google Scholar]
- Salas M. L., Kuznar J., Viñuela E. Polyadenylation, methylation, and capping of the RNA synthesized in vitro by African swine fever virus. Virology. 1981 Sep;113(2):484–491. doi: 10.1016/0042-6822(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Dales S. Biogenesis of poxviruses: identification of four enzyme activities within purified Yaba tumor virus. Virology. 1971 Sep;45(3):797–801. doi: 10.1016/0042-6822(71)90198-x. [DOI] [PubMed] [Google Scholar]
- Soloski M. J., Esteban M., Holowczak J. A. DNA-binding proteins in the cytoplasm of vaccinia virus-infected mouse L-cells. J Virol. 1978 Jan;25(1):263–273. doi: 10.1128/jvi.25.1.263-273.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Thomas V., Flores L., Holowczak J. A. Biochemical and electron microscopic studies of the replication and composition of milker's node virus. J Virol. 1980 Apr;34(1):244–255. doi: 10.1128/jvi.34.1.244-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODROOFE G. M., FENNER F. Serological relationships within the poxvirus group: an antigen common to all members of the group. Virology. 1962 Mar;16:334–341. doi: 10.1016/0042-6822(62)90255-6. [DOI] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylation of newly synthesized viral messenger RNA by an enzyme in vaccinia virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3014–3018. doi: 10.1073/pnas.71.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Kuenzle C. C., Wyler R. High C + G content in parapoxvirus DNA. J Gen Virol. 1979 Apr;43(1):231–234. doi: 10.1099/0022-1317-43-1-231. [DOI] [PubMed] [Google Scholar]
- Wittek R. Organization and expression of the poxvirus genome. Experientia. 1982 Mar 15;38(3):285–297. doi: 10.1007/BF01949349. [DOI] [PubMed] [Google Scholar]