INTRODUCTION
Obesity is now considered a worldwide epidemic. In the US, more than 30% of the adult population is currently obese, representing a two-fold increase since 1980 [1,2]. Concomitant with this burgeoning obesity epidemic has been a significant increase in obesity-associated diseases, most notably type 2 diabetes and cardiovascular disease [1,3].
Non-alcoholic fatty liver disease (NAFLD) is a newly emerging obesity-related disorder characterized by fatty infiltration of the liver in the absence of chronic alcohol consumption [4–6]. Similar to obesity, the prevalence of NAFLD has nearly doubled since 1980 [4,6,7]. Data from the most recent National Health and Nutrition Examination Survey (NHANES 1999–2002) suggest that the current prevalence of NAFLD is approximately 8.9% of the US population, as indicated by elevated levels of serum alanine aminotransferase (ALT) [8]. Diagnosis based solely on ALT levels, however, has been shown to underestimate the prevalence of NAFLD when compared to liver biopsies and radiographic techniques (e.g. magnetic resonance spectroscopy and computed tomography) [9,10]. Using these latter techniques, it has been estimated that NAFLD may affect 25–30% of the general population and up to 80% of obese and diabetic individuals [11]. Perhaps most alarming, NAFLD is emerging as a common pediatric disease, afflicting approximately 3–9% of all children in the US and up to 50% of obese children [12].
Recent data have demonstrated that NAFLD is closely associated with visceral adiposity, dyslipidemia and insulin resistance, and has been described as the hepatic component of the metabolic syndrome [6]. NAFLD ranges from fat accumulation in the liver (steatosis), to steatosis accompanied by inflammation and necrosis with or without fibrosis (non-alcoholic steatohepatitis or NASH), to end-stage liver disease [5,7]. Individuals with NAFLD often remain asymptomatic for decades. This indolent nature of NAFLD has contributed to an under-appreciation of its potential hazards [5]. However, NAFLD is now recognized as the most common cause of chronic liver enzyme elevations and cirrhosis [5,13], and more recent data suggest that the disease is independently associated with the development of cardiovascular disease and overall- and obesity-related mortality [14].
In light of the increasing prevalence and health consequences of NAFLD, there is a critical need to identify the mechanisms that mediate the development and progression of the disease. Research over the last decade has greatly enhanced our understanding of the disease in this regard, although numerous questions remain unanswered. For example, what metabolic abnormalities initiate the development of NAFLD. Also, what biochemical processes mediate the transition from simple steatosis to NASH. The current review will focus primarily on data from our laboratory and elsewhere examining the potential role of fatty acid composition in the progression of the disease. A putative role for the endoplasmic reticulum (ER) in the development and progression of NAFLD will also be discussed. Finally, we will compare and contrast the role of fatty acid composition in the pathophysiology of NAFLD with that of alcoholic fatty liver disease (AFLD), a disease histologically identical to NAFLD but with some intriguing differences.
TWO-HIT HYPOTHESIS
The current working model explaining the pathogenesis of NAFLD is the “two-hit” hypothesis, first proposed by Day et al. in 1998 [15]. According to this hypothesis, steatosis represents the “first hit”, which increases the vulnerability of the liver to various “second hits” that in turn lead to the inflammation, fibrosis and cellular death characteristic of NASH. Consistent with this hypothesis, administration of variously proposed second hits (e.g. endotoxin and pro-oxidants) results in significantly greater liver damage and lethality in obese mice with fatty liver compared to lean mice with healthy livers [16–18]. Furthermore, in humans, the severity of steatosis is one of the strongest predictors of the development of NASH [19].
Several factors have been suggested to constitute the second hit(s), most notably oxidative stress, pro-inflammatory cytokines and gut-derived bacterial endotoxin [4,20–22]. A detailed discussion of each of these putative factors is beyond the scope of this paper and is available in recent reviews [4,20–22]. It is important to note here, however, that these mechanisms are not mutually exclusive; but instead, likely act in a coordinated and cooperative manner to hasten the development and progression of NASH. For example, excess adiposity is associated with increased proinflammatory cytokines and oxidative stress, as well as an exaggerated inflammatory response to endotoxin administration [23]. Once generated, cytokines can cause direct liver damage or act indirectly by increasing oxidative stress, which in turn can also directly impair liver function or act indirectly by perpetuating the inflammatory response [13]. Therefore, in environments conducive to the generation of various second hits (e.g. obesity), a perpetuating cycle of insults may cause liver injury and culminate in NASH and, over time, end-stage liver disease.
One important aspect of the two hit hypothesis is that steatosis per se is not causal in the development of NASH; but rather, it sensitizes the liver to the damaging effects of second hits such that stressors innocuous to a healthy liver lead to the development of NASH in the steatotic liver. As will be discussed, however, an increasing body of literature suggests that the deposition of fat in the liver, and more specifically the type of fat that is deposited, may in fact directly damage the liver and precipitate the development of NASH.
LIPIDS AND NASH
Hepatocyte apoptosis is a salient feature and independent predictor of NASH [24,25]. In 1998, Unger et al. [26,27], first introduced the concept of lipoapoptosis, whereby over accumulation of lipids in non-adipose tissues leads to cell dysfunction and death. More recent data collected in various experimental models suggest that lipid-induced cell toxicity and apoptosis is specific to or made more severe by saturated fatty acids [28–32]. These data predict that the presence of increased circulating and/or hepatic saturated fatty acids, but not polyunsaturated fatty acids, may promote the development and progression of liver damage, in part via activation of apoptosis. Recent studies by our laboratory and others have tested this prediction [33–37].
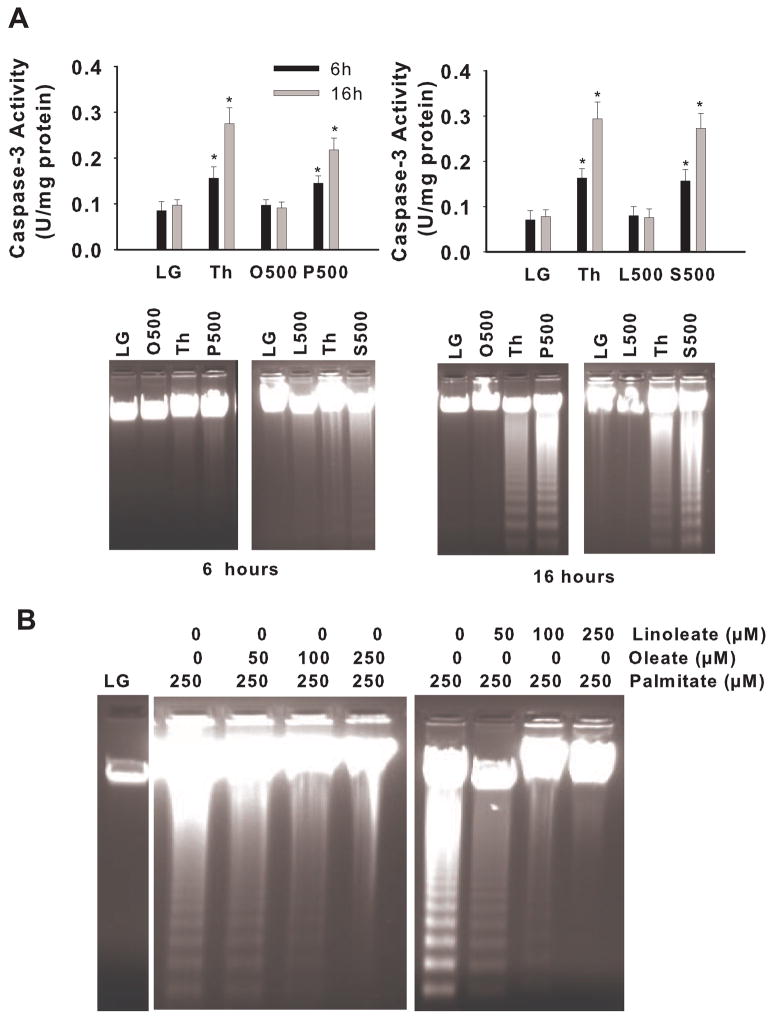
To examine the ability of individual fatty acids to induce apoptosis in liver cells, we exposed H4IIE hepatoma cells to either saturated (palmitate or stearate) or unsaturated (oleate or linoleate) fatty acids. Only palmitate and stearate increased caspase-3 activity and induced DNA fragmentation (Fig. 1A). Inclusion of the general caspase inhibitor Z-Val-Ala-Asp-fluoromethylketone prevented palmitate and stearate-induced DNA laddering, demonstrating that saturated fatty acid-induced apoptosis was caspase-dependent. Notably, co-incubation of palmitate with oleate or linoleate reduced palmitate-mediated apoptosis (Fig. 1B). This latter finding is consistent with previous data in pancreatic β cells, Chinese hamster ovary cells (CHO), and cardiomyocytes and suggests that the ratio of saturated-to-unsaturated fatty acids in cells is an important determinant of cell viability [30,34,38,39].
Figure 1.
Caspase activity and DNA fragmentation in H4IIE liver cells. A) Caspase-3 activity and DNA fragmentation were measure in liver cells following 6 or 16 hours of exposure to a control media (LG) or a control media supplemented with thapsigargin (Th, positive control), oleate at 500 μM (O500), palmitate at 500 μM (P500), linoleate at 500 μM (L500), or stearate at 500 μM (S500). B) DNA fragmentation was measured in liver cells following 16 hours of exposure to control media (LG) or control media supplemented with the noted concentrations of fatty acids. *, significantly different from LG and O500 or LG and L500 (p<0.05) [34].
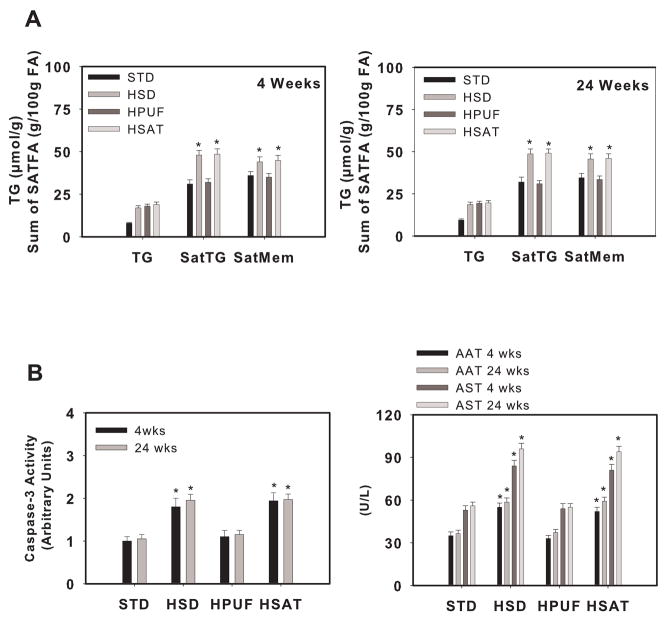
To examine whether an increased ratio of saturated-to-unsaturated fatty acids could induce liver injury in vivo, we utilized dietary models of hepatic steatosis [40,41]. Male Wistar rats were fed diets enriched with starch (STD), sucrose (HSD), polyunsaturated fat (HPUFA), or saturated fat (HSAT) for 1,4 or 24 weeks (only 4 and 24 wk data are shown in figures) [33]. Liver triglycerides were increased to a similar extent in HSD, HPUFA, and HSAT compared with STD at 4 and 24 weeks; however, saturated fatty acid content of triglycerides and microsomal membranes was increased in HSD and HSAT compared with HPUFA (Fig. 2A). Liver caspase-3 activity and plasma markers of liver injury were significantly higher in HSD and HSAT compared to STD and HPUFA (Fig. 2B). In addition, HSD and HSAT were characterized by reduced proliferative capacity following partial hepatectomy and increased liver injury in response to lipopolysaccharide compared to HPUFA. Thus, an increased saturated-to-unsaturated fatty acid ratio in the steatotic liver not only induced liver injury but also reduced proliferative capacity and increased the susceptibility of the liver to endotoxin. Importantly, increased liver injury in these dietary models was observed independently of differences in cytokines and insulin action. These data are consistent with the notion that the composition of fatty acids delivered to and stored within the liver is an important determinant of liver cell integrity, and potentially an independent risk factor for progression to NASH.
Figure 2.
Liver triglycerides, saturated fatty acid composition, caspase-3 activity and liver enzymes in dietary models of hepatic steatosis. Rats were fed a high starch (STD), high sucrose (HSD), high polyunsaturated fat (HPUF) or high saturated fat (HSAT) diet for 4 or 24 weeks. A) Liver triglyceride (TG) concentration and the sum of saturated fatty acids in triglycerides (SatTG) and microsomal membranes (SatMem). B) Liver caspase-3 activity and plasma concentrations of alanine aminotransferase (AAT) and aspartate aminotransferase (AST). *, significantly different from STD and HPUF (p<0.05) [33].
INTRACELLULAR SIGNALS MEDIATING SATURATED FATTY ACID-INDUCED TOXICITY
Despite unequivocal evidence that saturated fatty acids induce apoptosis in a number of cell types [28–32,38], including liver and hepatocytes [33,34,37], the mechanisms by which they do so are unclear. Ceramide accumulation, which can occur via enhanced de novo synthesis using palmitate or increased sphingomyelin breakdown, has been linked to both insulin resistance and apoptosis [42–45]. In pancreatic β cells and bovine retinal pericytes, saturated fatty acids not only increase ceramide levels, but inhibition of ceramide production prevents saturated fatty acid-induced apoptosis [26,46]. To determine the role of ceramide in saturated fatty acid-mediated apoptosis in liver cells, we incubated H4IIE cells with palmitate in the absence or presence of the ceramide synthetase inhibitor fumonisin B1 [34]. Palmitate significantly increased ceramide concentration in the absence of fumonisin B1, and the presence of fumonisin B1 prevented this increase. However, the presence of fumonisin B1 did not reduce palmitate-mediated apoptosis. These data are consistent with previous findings in CHO cells [29], and suggest that intracellular mediators of saturated fatty acid-induced apoptosis are cell specific, and that factors other than ceramide mediate the apoptotic effect in the liver.
It has been suggested that the accumulation of intrahepatic fatty acids can promote redox imbalance and the formation of reactive oxygen intermediates. A study performed in CHO cells demonstrated that palmitate-induced apoptosis required the generation of reactive intermediates [29]. In addition, other studies have found that reactive intermediates play a primary role in the activation stage of apoptosis [47–50]. Preliminary data (unpublished observations) from our laboratory suggest that both α-tocopherol (200 μM) and taurine (1%) reduce, but do not prevent, saturated fatty acid-induced apoptosis. Thus, other as yet unidentified intracellular signals, in addition to reactive intermediates, contribute to saturated fatty acid-induced apoptosis in liver cells.
The mitogen-activated protein kinase family of proteins is critical for the cellular response to a variety of stresses [51,52]. In particular, c-Jun NH2 terminal kinase (JNK) has emerged as a central metabolic regulator in obesity-related insulin resistance, appears to be a direct target of ceramide, and is activated by lipids [37,43,53,54]. Furthermore, a recent study demonstrated that saturated fatty acid-induced apoptosis in both primary mouse hepatocytes and HepG2 cells was mediated in part by activation of JNK [37]. Data from our laboratory supports an important role for JNK in saturated fatty acid-induced apoptosis in the liver [36]. In this study, we examined insulin-mediated protection against saturated fatty acid-induced apoptosis in the rat hepatoma cell line, H4IIE and in primary rat hepatocytes [36]. Cells were provided a control media (no fatty acids) or the same media containing 250 μmol/L of albumin-bound oleate or palmitate for 16 h. Insulin concentrations were 0, 1, 10 or 100 nM. Palmitate, but not oleate, activated caspase-3 and induced DNA fragmentation in the absence of insulin. Insulin reduced palmitate-mediated activation of caspase-3 and DNA fragmentation in a dose-dependent manner. PI3-kinase inhibitors abolished these effects of insulin. Palmitate, but not oleate, increased JNK activity in the absence of insulin. Insulin or SP600125, a chemical inhibitor of JNK, blocked palmitate-mediated activation of JNK and reduced apoptosis. These data not only support a role for JNK in palmitate-mediated apoptosis, but also suggest that insulin is an important determinant of saturated fatty acid-induced apoptosis in liver. Thus, these findings may have implications for fatty acid-mediated liver cell injury in insulin deficient and/or resistant states.
THE ENDOPLASMIC RETICULUM IS A TARGET FOR SATURATED FATTY ACIDS
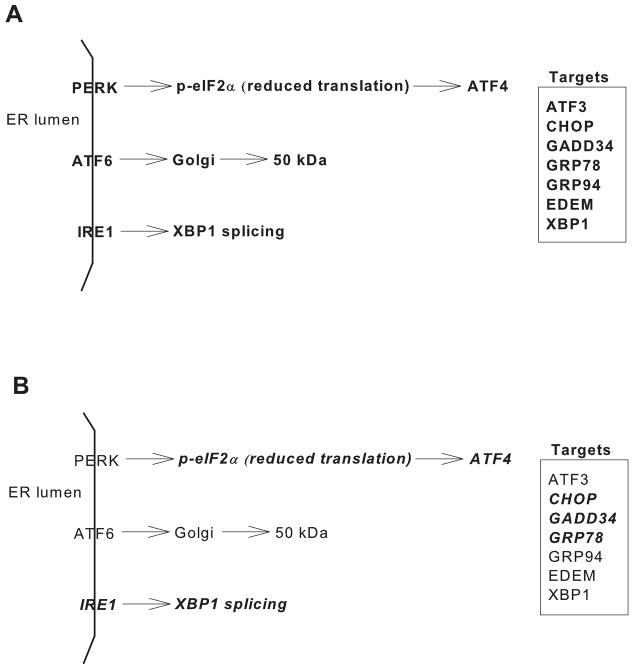
The endoplasmic reticulum (ER) is one of the largest cellular organelles, its membranes representing as much as one half of the total membranes in a cell [55]. The ER lumen comprises over 10% of the cell volume and is characterized by a unique environment that includes the highest concentration of calcium within the cell and an oxidative environment to support disulfide bond formation [55,56]. An essential function of the ER is the proper assembly of proteins that are ultimately destined for intracellular organelles and the cell surface. The status of protein assembly and folding is monitored and relayed to the cytosol and nucleus by the unfolded protein response (UPR) [56–59]. A variety of stressors, including loss of the luminal oxidizing environment, imbalance in calcium homeostasis, and aberrant N-glycosylation disrupt ER homeostasis and lead to the accumulation of unfolded proteins and protein aggregates in the ER lumen, both of which can be detrimental to cell survival. Disruption of ER homeostasis, collectively termed ER stress, activates the UPR. In mammals, ER stress is sensed and the UPR activated by three ER transmembrane proteins, PERK (RNA-dependent protein kinase-like ER eukaryotic initiation factor-2α kinase), ATF6 (activating transcription factor 6), and IRE1 (inositol-requiring ER-to-nucleus signaling protein 1) (Fig. 3A). PERK activation leads to phosphorylation of the α-subunit of the translation initiation factor eIF2 and subsequent attenuation of translation initiation, and increases the expression and selective translation of activation transcription factor 4 (ATF4). Increased expression of GADD34, a member of the growth arrest and DNA damage family of proteins, is involved in dephosphorylation of eIF2α and, therefore, reversal of translational attenuation. Upon UPR activation, ATF6 is transported to the Golgi where it is cleaved and subsequently migrates to the nucleus, as a 50 kDa fragment, and activates transcription of UPR target genes. Activation of IRE1 promotes the splicing of X-box-binding protein-1 (XBP1) mRNA and subsequent transcription of molecular chaperones (e.g. GRP78) and genes involved in ER-associated degradation [(e.g., ER mannosidase (EDEM)] [56,60–66]. Thus, activation of the UPR serves to attenuate global protein synthesis and enhance the capacity for protein folding and degradation. Failure of the UPR to re-establish ER homeostasis can lead to programmed cell death [56,67].
Figure 3.
A) Schematic diagram depicting major components of the unfolded protein response as described in text. B) Schematic diagram depicting (bold and italics) the components of the UPR that are known to be activated in response to long chain saturated fatty acids.
Several studies have linked ER dysfunction and the UPR to impairments in glucose homeostasis and diabetes. For example, PERK −/− mice develop diabetes due to a rapid and progressive decline in endocrine and exocrine pancreatic function [68]. Conversely, mice with a homozygous mutation of serine 51 on eIF2α die within 18 h of birth as a result of hypoglycemia and impaired induction of genes involved in hepatic gluconeogenesis [69]. Programmed cell death in response to ER stress is mediated, in part, through transcriptional activation of CCAAT/enhancer binding homologous protein (CHOP) [70,71]. Targeted disruption of the CHOP gene in Akita mice, a mouse line that spontaneously develops hyperglycemia with reduced β-cell mass, delayed the onset of diabetes [72]. Thus, it has been proposed that chronic disruption of ER homeostasis may contribute to the attrition of β-cell function and to impaired regulation of glucose homeostasis in diabetes [73–75].
An elegant study also identified the UPR as a molecular link between obesity and deterioration of insulin action in liver and adipose tissue [76]. However, this study did not examine how obesity led to disruption of ER homeostasis. The ER membrane is characterized by a low concentration of cholesterol and a high concentration of polyunsaturated fatty acids, a lipid environment consistent with a “disordered” membrane [77]. Recent evidence has demonstrated that cholesterol loading activates the UPR and induces apoptosis in macrophages, suggesting that the UPR senses changes to the membrane cholesterol environment [78]. To determine whether the UPR senses changes in the fatty acid environment, we exposed H4IIE hepatoma cells to either saturated (palmitate or stearate) or unsaturated (oleate or linoleate) fatty acids [34–36]. Incubation with palmitate or stearate resulted in a significant increase in the expression of biochemical markers of the UPR (GRP78, ATF4, GADD34, CHOP) and XBP1 splicing at concentrations ranging from 100 to 500 μM [34,35]. Saturated fatty acid-activation of the UPR preceded apoptosis [34,35]. Neither oleate nor linoleate altered any markers of UPR activation, and co-incubation of palmitate with oleate or linoleate reduced palmitate-induced UPR activation [34]. To determine whether saturated fatty-acids compromise ER homeostasis in vivo, we measured several markers of ER stress in the aforementioned study in which male Wistar rats were fed diets enriched with starch (STD), sucrose (HSD), polyunsaturated fat (HPUFA), or saturated fat (HSAT) for 1,4 or 24 weeks (Fig. 2) [33]. Livers and hepatocytes from HSD and HSAT rats, but not STD or HPUFA, were characterized by the presence of spliced XBP-1 mRNA and increased GRP78 and CHOP protein [33]. These results suggest that the UPR may sense and respond to the fatty acid environment and also indicate that the ratio of saturated to unsaturated fatty acids may be an important determinant of hepatic ER homeostasis. Future studies are necessary to determine whether ER stress and activation of the UPR are causally linked to saturated fatty acid-induced apoptosis and liver injury.
It is presently unclear how saturated fatty acids induce ER stress. Saturated fatty acids disrupt ER homeostasis and induce apoptosis in liver cells via mechanisms that do not appear to involve ceramide accumulation [34]. Several studies suggest that saturated fatty acids-induce cytotoxicity and/or disrupt ER homeostasis via selective, structural effects to the ER. For example, in vitro data suggest that palmitoyl CoA can inhibit ER assembly and propagate ER membrane fission [79]. In pancreatic β-cells, Busch et al [80] demonstrated that saturation per se provoked cytotoxicity. In INS1 cells, palmitate was converted in the ER to solid tripalmitin, thus induction of ER stress and apoptosis was attributed to physicochemical properties of these “saturated” triglycerides [81]. In a highly innovative series of experiments, Borradaile et al. [82] demonstrated that palmitate-induced ER stress in CHO cells and H9c2 cardiomyocytes was associated with the rapid incorporation of palmitate into lipid components of the rough ER followed by disruption of ER structure and function. Thus, it is possible that the trafficking of saturated fatty acids to the ER membrane may be an important determinant of ER homeostasis [38,82]. Further work is necessary to determine whether selective lipid trafficking to the ER is a component of saturated fatty acid-induced ER stress in hepatocytes.
A DILEMMA: SATURATED FATTY ACIDS ARE PROTECTIVE IN ALCOHOL-INDUCED FATTY LIVER DISEASE
Alcoholic fatty liver disease (AFLD) affects nearly 50% of alcohol abusers and is a major cause of illness and death among these individuals [83]. AFLD shares numerous similarities with NAFLD. The natural history of both diseases is characterized by an initial over accumulation of fat in the liver, which progresses in some individuals to steatohepatitis and cirrhosis. Obesity and insulin resistance, the two principal risk factors for NAFLD, appear to also increase the incidence of all stages of AFLD in heavy drinkers [84,85]. Histologically, the two diseases are indistinguishable, and pathologically, the two diseases appear to share at least two mechanistic pathways, oxidative stress and pro-inflammatory cytokines [86,87].
Recent evidence also suggests that AFLD is associated with ER stress. Using a murine model of intragastric ethanol feeding, Ji et al., found that the development of steatosis following 6 weeks of ethanol ingestion was accompanied by increases in several ER stress-related proteins, including GRP78, GRP94, CHOP and caspase 12 [88,89]. The induction of ER stress was mediated, in part, by hyperhomocysteinemia, and was independent of TNF-α. In a subsequent study by the same group, CHOP null mice were protected against ethanol-induced apoptosis despite the development of fatty liver, suggesting a causal role for this transcription factor in alcohol-related cell death [90].
Despite the numerous similarities between AFLD and NAFLD, some notable differences exist. One of the more intriguing relates to the role of fatty acid composition in the development of liver injury. In liver and hepatocytes not exposed to alcohol, saturated fatty acids appear to promote apoptosis and liver injury [33,34,37,91]. In contrast, the opposite appears to be true in AFLD; that is, saturated fatty acids reduce/prevent and unsaturated fats promote alcohol-related liver injury [91–94]. The protective effect of saturated fatty acids was initially observed in an intragastric rat feeding model of alcoholic liver disease, in which ethanol in combination with a liquid diet containing corn oil produced severe liver pathology, whereas equicaloric liquid diets containing either beef tallow or lard produced no or minimal to moderate pathology, respectively [91]. In fact, this study suggested that linoleic acid may be an essential factor in the development of AFLD. Notably, the protective effects of saturated fatty acids in this model of ALFD appear to be associated with a reduction of steatosis via a combination of reduced fatty acid synthesis and increased fatty acid oxidation and lipid export [94].
The mechanism(s) by which saturated fats protect against alcohol-induced liver injury are unclear. A large body of literature supports a role for oxidative stress in AFLD. Cytochrome P450 2E1, which assists in alcohol metabolism during excessive or chronic alcohol consumption, can contribute to oxidative stress via formation of oxygen radicals and lipid peroxidation [95]. Therefore, it is of note that saturated fats reduced alcohol-induced lipid peroxidation and upregulation of CYP2E1 [96]. However, upregulation of CYP2E1 by dietary saturated fat has not been a universal finding [94]. Saturated fats have also been shown to reduce proinflammatory mediators, including TNF-α, cyclooxygenase-2 and NFκB [92,93]. It has also been suggested that dietary saturated fat alleviates ALFD, in part, via upregulation of adiponectin expression and production in adipose tissue [97]. These changes in adiponectin, in turn, may contribute to the enhancement of fatty acid oxidation and thus reduced steatosis.
Collectively, the existing data provide compelling evidence that saturated fatty acids protect against the development and progression of AFLD. The mechanisms by which they elicit these protective effects are unclear, although reductions in the magnitude of steatosis, oxidative stress and inflammatory pathway activation appear to be involved. Since long chain saturated fatty acids promote oxidative stress and activate inflammatory pathways in cells and tissues not exposed to alcohol [29,37,46,98–100], it seems likely that the presence of alcohol alters metabolism of specific fatty acids within tissues. Subsequent studies that directly compare the effect of saturated fatty acids in models of alcoholic- and nonalcoholic fatty liver disease are needed to address these discrepancies.
SUMMARY AND PERSPECTIVE
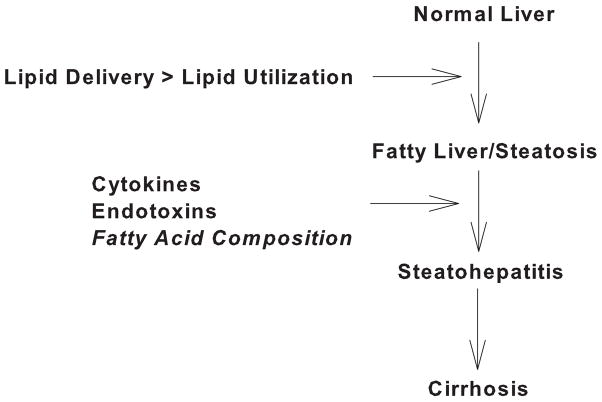
NAFLD has emerged as a serious and widespread obesity-related disorder. The full spectrum of NAFLD ranges from hepatic fat accumulation in the absence of major histological aberrations to fat accumulation accompanied by fibrosis and necrosis. The two-hit hypothesis postulates that hepatic fat accumulation per se is not injurious, but rather, secondary insults (e.g. ROS, inflammatory cytokines) imposed upon the fatty liver are necessary for progression to steatohepatitis. However, a growing body of literature strongly suggests that hepatic fatty acid composition may impact the degree of liver injury and therefore disease progression. We propose that an increased ratio of saturated-to-unsaturated fatty acids delivered to or stored within the liver may contribute to progression from simple steatosis to NASH. Therefore, within the context of the two-hit hypothesis, saturated fatty acids may represent an intrinsic second hit that hastens the development of NASH.
It is important to emphasize that cellular and murine models of NAFLD are far removed from the free living conditions in which people typically develop the disease. Thus, it is important to determine if the cytotoxic effects of saturated fatty acids observed in animal and cell culture models are relevant to the development of the disease in humans. In this context, it has recently been found in NAFLD patients that a considerable portion of hepatic triglycerides are derived from the diet [101]. Given that saturated fatty acids and simple sugars constitute a significant portion of the American diet [102–105], and that at least some patients with NASH consume more saturated fat and carbohydrate and less unsaturated fats than healthy weight-matched controls [106–108], it is reasonable to speculate that the amount of saturated fat in the liver of NAFLD patients that progress to NASH may be increased. Indeed, the presence of increased saturated fatty acids in serum cholesterol esters has been observed in individuals with type 2 diabetes [109]. In future studies, it will be important to examine the relationship between circulating and intrahepatic fatty acid composition and liver damage in patients with NAFLD.
Figure 4.
Schematic diagram depicting disease progression in NAFLD and hypothesized second hits.
Acknowledgments
The authors would like to acknowledge the Pagliassotti Lab and support from the NIH, DK072017
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 4.Festi D, Colecchia A, Sacco T, Bondi M, Roda E, Marchesini G. Hepatic steatosis in obese patients: clinical aspects and prognostic significance. Obesity Reviews. 2004;5:27–42. doi: 10.1111/j.1467-789x.2004.00126.x. [DOI] [PubMed] [Google Scholar]
- 5.Clark JM, Diehl A. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–3004. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- 6.Akbar DH, Kawther AH. Non-alcoholic fatty liver disease and metabolic syndrome: what we know and what we don’t know. Med Sci Monit. 2006;12:RA23–26. [PubMed] [Google Scholar]
- 7.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 9.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 10.Mofrad PS, Sanyal AJ. Nonalcoholic fatty liver disease. MedGenMed. 2003;5:14. [PubMed] [Google Scholar]
- 11.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 12.Papandreou D, Rousso I, Mavromichalis I. Update on non-alcoholic fatty liver disease in children. Clin Nutr. 2007;26:409–415. doi: 10.1016/j.clnu.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Abdelmalek MF, Diehl A. Mechanisms underlying nonalcoholic steatohepatitis. Drug Discovery Today Disease Mechanisms. 2006;3:479–488. [Google Scholar]
- 14.Targher G, Bertolini L, Poli F, Rodella S, Scala L, Tessari R, Zenari L, Falezza G. Nonalcoholic Fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes. 2005;54:3541–3546. doi: 10.2337/diabetes.54.12.3541. [DOI] [PubMed] [Google Scholar]
- 15.Day CP, James O. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 16.Yang SQ, Lin HZ, Mandal AK, Huang J, Diehl AM. Disrupted signaling and inhibited regeneration in obese mice with fatty livers: Implications for nonalcoholic fatty liver disease pathophysiology. Hepatology. 2001;34:694–706. doi: 10.1053/jhep.2001.28054. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Kiyosawa N, Kumagai K, Manabe S, Matsunuma N, Yamoto T. Molecular mechanism investigation of cycloheximide-induced hepatocyte apoptosis in rat livers by morphological and microarray analysis. Toxicology. 2006;219:175–186. doi: 10.1016/j.tox.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am J Physiol. 1999;276:R136–142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 19.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 20.Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28:1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Clark J, Diehl A. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 22.Harrison SA, Kadakia S, Lang KA, Schenker S. Nonalcoholic steatohepatitis: what we know in the new millennium. Am J Gastroenterol. 2002;97:2714–2724. doi: 10.1111/j.1572-0241.2002.07069.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominant features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 25.Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in Nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 26.Shimabukuro M, Zhou Y-T, Levi M, Unger RH. Fatty acid-induced B cell apoptosis: A link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/s1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 28.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 29.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 30.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–1394. [PubMed] [Google Scholar]
- 31.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and unsaturated fatty acids on B-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 33.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 34.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am J Physiol Endocrinol Metab. 2006;291:E275–E281. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 35.Wei Y, Wang D, Pagliassotti MJ. Saturated fatty acid-mediated endoplasmic reticulum stress and apoptosis are augmented by trans-10, cis-12-conjugated linoleic acid in liver cells. Mol Cell Biochem. 2007 doi: 10.1007/s11010-007-9461-2. [DOI] [PubMed] [Google Scholar]
- 36.Pagliassotti MJ, Wei Y, Wang D. Insulin Protects Liver Cells from Saturated Fatty Acid-Induced Apoptosis via Inhibition of c-Jun NH2 Terminal Kinase Activity. Endocrinology. 2007;148:3338–3345. doi: 10.1210/en.2006-1710. [DOI] [PubMed] [Google Scholar]
- 37.Malhi H, Bronk SF, Werneburg NW, Gores GJ. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J Biol Chem. 2006;281:12093–12101. doi: 10.1074/jbc.M510660200. [DOI] [PubMed] [Google Scholar]
- 38.Listenberger LJ, Han X, Lewis SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharroubi I, Ladriere L, Cardozo AK, Dogusan Z, Cnop M, Eizirik DL. Free fatty acids and cytokines induce pancreatic B-cell apoptosis by different mechanisms: Role of nuclear factor-KB and endoplasmic reticulum stress. Endocrinology. 2004;145:5087–5096. doi: 10.1210/en.2004-0478. [DOI] [PubMed] [Google Scholar]
- 40.Pagliassotti MJ, Prach PA, Koppenhafer TA, Pan DA. Changes in insulin action, triglycerides, and lipid composition during sucrose feeding in rats. Am J Physiol. 1996;271:R1319–1326. doi: 10.1152/ajpregu.1996.271.5.R1319. [DOI] [PubMed] [Google Scholar]
- 41.Commerford SR, Ferniza JB, Bizeau ME, Thresher JS, Willis WT, Pagliassotti MJ. Diets enriched in sucrose or fat increase gluconeogenesis and G-6-Pase but not basal glucose production in rats. Am J Physiol Endocrinol Metab. 2002;283:E545–555. doi: 10.1152/ajpendo.00120.2002. [DOI] [PubMed] [Google Scholar]
- 42.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regulatory Integrative Comp Physiol. 2005;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 43.Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP. Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res. 2004;64:7852–7856. doi: 10.1158/0008-5472.CAN-04-1552. [DOI] [PubMed] [Google Scholar]
- 44.Willaime-Morawek S, Brami-Cherrier K, Mariani J, Caboche J, Brugg B. C-Jun N-terminal kinases/c-Jun and p38 pathways cooperate in ceramide-induced neuronal apoptosis. Neuroscience. 2003;119:387–397. doi: 10.1016/s0306-4522(02)00996-x. [DOI] [PubMed] [Google Scholar]
- 45.Ruvolo PP. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol Res. 2003;47:383–392. doi: 10.1016/s1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- 46.Cacicedo JM, Benjachareowong S, Chou E, Ruderman NB, Ido Y. Palmitate-induced apoptosis in cultured bovine retinal pericytes: roles of NAD(P)H oxidase, oxidant stress, and ceramide. Diabetes. 2005;54:1838–1845. doi: 10.2337/diabetes.54.6.1838. [DOI] [PubMed] [Google Scholar]
- 47.Buttke TM, Sandstrom PA. Redox regulation of programmed cell death in lymphocytes. Free Radic Res. 1995;22:389–397. doi: 10.3109/10715769509147548. [DOI] [PubMed] [Google Scholar]
- 48.Jacobson MD. Reactive oxygen species and programmed cell death. Trends Biochem Sci. 1996;21:83–86. [PubMed] [Google Scholar]
- 49.Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252:1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 50.Aronis A, Madar Z, Tirosh O. Mechanism underlying oxidative stress-mediated lipotoxicity: Exposure of J774.2 macrphages to triacylglycerols facilitates mitochondrial reactive oxygen species production and cellular necrosis. Free Rad Biol & Med. 2005;38:1221–1230. doi: 10.1016/j.freeradbiomed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 51.Boldt S, Kolch W. Targeting MAPK signaling: Prometheus’ fire or Pandora’s box. Curr Pharm Des. 2004;10:1885–1905. doi: 10.2174/1381612043384420. [DOI] [PubMed] [Google Scholar]
- 52.Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 54.Czaja MJ. The future of GI and LIver Research: Editorial Perspectives III. JNK/AP-1 regulation of hepatocyte death. Am J Physiol Gastrointest Liver Physiol. 2002;284:G875–G879. doi: 10.1152/ajpgi.00549.2002. [DOI] [PubMed] [Google Scholar]
- 55.Pahl HL. Signal transduction from the endoplasmic reticulum to the cell nucleus. Physiol Rev. 1999;79:683–701. doi: 10.1152/physrev.1999.79.3.683. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 59.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 60.Zhang K, Kaufman RJ. Signaling the unfolded protein response from the endoplasmic reticulum. J Biol Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 61.Shen J, Prywes R. ER stress signaling by regulated proteolysis of ATF6. Methods. 2005;35:382–389. doi: 10.1016/j.ymeth.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thuerauf DJ, Morrison L, Glembotski CC. Opposing roles for ATF6α and ATF6β in endoplasmic reticulum stress response gene induction. J Biol Chem. 2004;279:21078–21084. doi: 10.1074/jbc.M400713200. [DOI] [PubMed] [Google Scholar]
- 64.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 65.Hong M, Luo S, Baumeister P, Huang J-M, Gogia RK, Li M, Lee AS. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response. J Biol Chem. 2004;279:11354–11363. doi: 10.1074/jbc.M309804200. [DOI] [PubMed] [Google Scholar]
- 66.Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 67.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 68.Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D. Diabetes mellitus and exocrine pancreatic dysfunction in Perk −/− mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 69.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 70.Kim SH, Hwang CI, Park WY, Lee JH, Song YS. GADD153 mediates celecoxib-induced apoptosis in cervical cancer cells. Carcinogenesis. 2006;27:1961–1969. doi: 10.1093/carcin/bgl027. [DOI] [PubMed] [Google Scholar]
- 71.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 72.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum-stress mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi Y, Taylor SI, Tan S-L, Sonenberg N. When translation meets metabolism: Multiple links to diabetes. Endocrine Reviews. 2003;24:91–101. doi: 10.1210/er.2002-0018. [DOI] [PubMed] [Google Scholar]
- 74.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006 doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Wang D, Wei Y, Schmoll D, Maclean KN, Pagliassotti MJ. Endoplasmic reticulum stress increases glucose-6-phosphatase and glucose cycling in liver cells. Endocrinology. 2006;147:350–358. doi: 10.1210/en.2005-1014. [DOI] [PubMed] [Google Scholar]
- 76.Ozcan U, Cao Q, Yilmaz E, Lee A-H, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun CZ, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 77.Spector AA, Yorek MA. Membrane lipid composition and cellular function. J Lipid Res. 1985;26:1015–1035. [PubMed] [Google Scholar]
- 78.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biology. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 79.Turner MD. Fatty acyl CoA-mediated inhibition of endoplasmic reticulum assembly. Biochim Biophys Acta. 2004;1693:1–4. doi: 10.1016/j.bbamcr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Busch AK, Gurisik E, Cordery DV, Sudlow M, Denyer GS, Laybutt DR, Hughes WE, Biden TJ. Increased fatty acid desaturation and enhanced expression of stearoyl coenzyme A desaturase protects pancreatic B-cells from lipoapoptosis. Diabetes. 2005;54:2917–2924. doi: 10.2337/diabetes.54.10.2917. [DOI] [PubMed] [Google Scholar]
- 81.Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 82.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 83.Mills SJ, Harrison SA. Comparison of the natural history of alcoholic and nonalcoholic Fatty liver disease. Curr Gastroenterol Rep. 2005;7:32–36. doi: 10.1007/s11894-005-0063-4. [DOI] [PubMed] [Google Scholar]
- 84.Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25:108–111. doi: 10.1002/hep.510250120. [DOI] [PubMed] [Google Scholar]
- 85.Raynard B, Balian A, Fallik D, Capron F, Bedossa P, Chaput JC, Naveau S. Risk factors of fibrosis in alcohol-induced liver disease. Hepatology. 2002;35:635–638. doi: 10.1053/jhep.2002.31782. [DOI] [PubMed] [Google Scholar]
- 86.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 87.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 88.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 89.Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. Hepatology. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- 90.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nanji AA, Mendenhall CL, French SW. Beef fat prevents alcoholic liver disease in the rat. Alcohol Clin Exp Res. 1989;13:15–19. doi: 10.1111/j.1530-0277.1989.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 92.Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Dannenberg AJ. Dietary saturated fatty acids reverse inflammatory and fibrotic changes in rat liver despite continued ethanol administration. J Pharmacol Exp Ther. 2001;299:638–644. [PubMed] [Google Scholar]
- 93.Nanji AA, Zakim D, Rahemtulla A, Daly T, Miao L, Zhao S, Khwaja S, Tahan SR, Dannenberg AJ. Dietary saturated fatty acids down-regulate cyclooxygenase-2 and tumor necrosis factor alfa and reverse fibrosis in alcohol-induced liver disease in the rat. Hepatology. 1997;26:1538–1545. doi: 10.1002/hep.510260622. [DOI] [PubMed] [Google Scholar]
- 94.Ronis MJ, Korourian S, Zipperman M, Hakkak R, Badger TM. Dietary saturated fat reduces alcoholic hepatotoxicity in rats by altering fatty acid metabolism and membrane composition. J Nutr. 2004;134:904–912. doi: 10.1093/jn/134.4.904. [DOI] [PubMed] [Google Scholar]
- 95.Morimoto M, Hagbjork AL, Nanji AA, Ingelman-Sundberg M, Lindros KO, Fu PC, Albano E, French SW. Role of cytochrome P4502E1 in alcoholic liver disease pathogenesis. Alcohol. 1993;10:459–464. doi: 10.1016/0741-8329(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 96.Nanji AA, Yang EK, Fogt F, Sadrzadeh SMH, Dannenberg AJ. Medium chain triglyerides and vitamin E reduce the severity of established experimental alcoholic liver disease. J Pharmacol Exp Ther. 1996;277:1694–1700. [PubMed] [Google Scholar]
- 97.You M, Considine RV, Leone TC, Kelly DP, Crabb DW. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology. 2005;42:568–577. doi: 10.1002/hep.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 99.Rachek LI, Musiyenko SI, Ledoux SP, Wilson GL. Palmitate induced mtDNA damage and apoptosis in L6 rat skeletal muscle cells. Endocrinology. 2006 doi: 10.1210/en.2006-0998. [DOI] [PubMed] [Google Scholar]
- 100.Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y, Zhang L, Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006 doi: 10.1016/j.lfs.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 101.Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bazzano LA, Serdula M, Liu S. Prevention of type 2 diabetes by diet and lifestyle modification. J Am Coll Nutr. 2005;24:310–319. doi: 10.1080/07315724.2005.10719479. [DOI] [PubMed] [Google Scholar]
- 103.Bizeau ME, Pagliassotti MJ. Hepatic adaptations to sucrose and fructose. Metabolism. 2005;54:1189–1201. doi: 10.1016/j.metabol.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 104.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005;63:133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 105.Bolton-Smith C, Woodward M. Dietary composition and fat to sugar ratios in relation to obesity. Int J Obes Relat Metab Disord. 1994;18:820–828. [PubMed] [Google Scholar]
- 106.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, Faga E, Silli B, Pagano G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 107.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, Foess-Wood L, Sherbondy MA, Conjeevaram HS. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247–2253. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 108.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, Emick D, Lok AS, Conjeevaram HS. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 109.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes. 1994;43:1353–1357. doi: 10.2337/diab.43.11.1353. [DOI] [PubMed] [Google Scholar]