Abstract
Protein Phosphatase 5 (PP5) is a unique member of the PPP family of serine/threonine phosphatases based on the presence of tetratricopeptide repeat (TPR) domains within its structure. Since its discovery, PP5 has been implicated in wide ranging cellular processes, including MAPK-mediated growth and differentiation, cell cycle arrest and DNA damage repair via the p53 and ATM/ATR pathways, regulation of ion channels via the membrane receptor for atrial natriuretic peptide, the cellular heat shock response as mediated by heat shock transcription factor, and steroid receptor signaling, especially glucocorticoid receptor (GR). Given this diversity of effects, the recent development of viable PP5-deficient mice was surprising and suggests that PP5 is a modulatory, rather than essential, factor in phosphorylation pathways. Here, we review the signaling involvement of PP5 in light of new findings and relate these activities to the structural features of the protein.
Keywords: phosphatase, tetratricopeptide repeat protein, PP5, Ppp5, Protein phosphatase 5, phosphoprotein, steroid receptor, glucocorticoid receptor, chaperone, HSP90, MAPK
Introduction
Although PP5 is the commonly accepted name of this important phosphatase, alternative names can be found in the literature, including Ppp5 (Zeke et al., 2005; Yong et al., 2007), and phosphoprotein phosphatase 5 (Russell et al., 1999). Identification of PP5 came late compared to other members of the PPP family of serine/threonine-specific phosphatases, such as PP1, PP2A, and PP2B – most likely due to its low basal phosphatase activity (Chen and Cohen, 1997). Using different methods, three laboratories are credited for the discovery of PP5 in 1994 (Becker et al., 1994; Chen et al., 1994; Chinkers, 1994); the Cohen laboratory provided the nomenclature, PP5 (Chen et al., 1994). Sequencing of PP5, and its yeast homolog PPT, showed the presence of TPR domains within their structures. Although many proteins use TPR domains as protein-protein interaction motifs, PP5 and PPT are the only phosphatases known to contain these structures. Not surprisingly, PP5 was later found to interact with Heat Shock Protein 90 (HSP90) (Chen et al., 1996), a molecular chaperone known to bind other TPR containing proteins, such as FKBP52, FKBP51 and Cyp40 (for review see (Pratt and Toft, 1997)). As a well-known function of HSP90 is to control the early stages of steroid receptor (SR) activity, PP5 has been actively studied with respect to its actions on steroid receptors. In this context, it should be noted that HSP90, although it exists as a dimer in SR complexes, only generates one TPR-binding site per receptor complex (Pratt and Toft, 1997). Thus, multiple SR complexes are known to exist based on TPR protein content. Therefore, PP5 is best viewed as part of a modulatory cartel of distinct TPR-containing HSP90 complexes whose principal and unique roles in regulation of SRs and other clients are still largely unknown.
Structure
A diagram showing pertinent features of PP5 and its isoforms is seen in Fig. 1. The phosphatase domain resides in the C-terminal region and contains all the relevant motifs of the PPP family of phosphatases (Becker et al., 1994; Chen et al., 1994; Chinkers, 1994). Key residues in the phosphatase domain responsible for binding the phosphate moeity of substrates are R275, N303, H304 and R400 (Swingle et al., 2004). Interestingly, sequence homology is low (approximately 40%) when comparing the phosphatase domain of PP5 to those of PP1, PP2A, or PP2B, prompting Cohen in 1994 to assign PP5 to its own subfamily (Chen et al., 1994). Contributing to its uniqueness, PP5 contains two other domains not found in the PPP family, a peptidyl-prolyl cis-trans isomerase-like (PPIase-like) domain and three consecutive TPR domains located in the N-terminal region. TPR domains are imperfect 34 amino acid sequences that mediate protein-protein interactions. The crystal structure of the PP5 TPR domain has been determined by Barford and co-workers (Das et al., 1998). The structure shows strong similarity to TPR regions of other proteins. Each TPR motif consists of a pair of antiparallel α-helices, with adjacent TPRs packing together to form a series of paired-helices, generating an amphipathic groove that serves as the binding surface. Probably, the best-known binding partner of PP5 is HSP90. PP5 binds the C-terminal EEVD sequence of HSP90, and four amino acids (K32, R74, K97, and R101) in the TPR domain of PP5 are essential for this interaction (Russell et al., 1999).
Fig. 1.
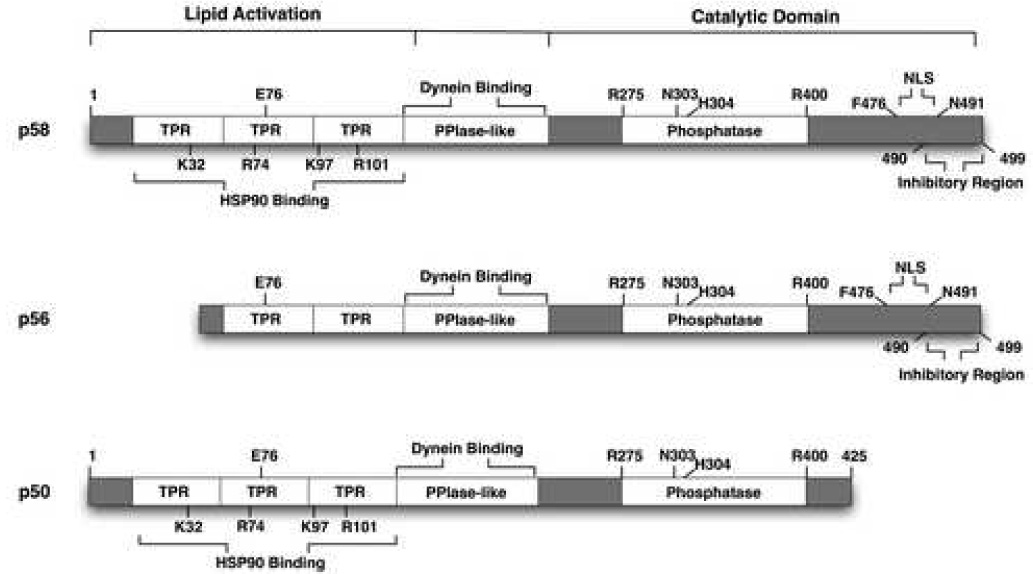
Diagrammatic representation of PP5 and its isoforms. See text for description of the functional domains and sites.
PP5, unlike other phosphatases, displays low phosphatase activity under normal conditions (Chen and Cohen, 1997) that may result from an inhibitory interaction between the C-terminus (residues 490–499) and the TPR domain (Kang et al., 2001). Residues E76 in the second TPR motif and Q495 in the C-terminus are critical to the inhibitory interaction (Kang et al., 2001). Such intrachain interactions may allow the TPR domain to mediate phosphatase activity by binding and shielding it from activation. The phosphatase domain can be activated when the TPR domains are bound or truncated from PP5 (Chen and Cohen, 1997). Normally PP5 is a 58kDa protein that binds HSP90. Truncated versions of PP5 give rise to two isoforms, p56 and p50 (Fig. 1). Proteolytic cleavage of the N-terminus generates the p56 isoform, whereas cleavage of the C-terminus produces p50 (Zeke et al., 2005). Cleavage to p50 removes a putative nuclear localization signal (NLS) at residues 476–491, generating an isoform that is found predominantly in the cytoplasm. Although an antibody specific to the catalytic domain can bind p50, it is still unclear if this isoform exhibits catalytic activity. However, p56 is likely to be catalytically active, as truncation of the TPR domain should cause derepression of the phosphatase domain. It is for this reason that stimulation of PP5 can occur with polyunsaturated fatty acids, such as arachidonic acid (Skinner et al., 1997), which bind the TPR domain of PP5 to initiate phosphatase activity.
Biological Function
A delicate balance of phosphorylation and dephosphorylation is essential to the maintenance of cellular responsiveness. In the thirteen years since its discovery, it is evident that PP5 is a multi-tasking regulator of this balance. Cellular functions impacted by PP5 include proliferation, migration, differentiation, electrolyte balance, apoptosis, survival, and DNA damage repair. Not surprisingly, PP5 is expressed in virtually all mammalian tissues, with particularly high levels in brain and neurons (Becker et al., 1994; Chinkers, 1994). Within the cell, PP5 is also broadly distributed; with early reports suggesting predominant localizaton to the nucleus (Chen et al., 1994; Chen and Cohen, 1997), while most recent reports find a strong cytoplasmic signal sometimes in association with microtubules (Galigniana et al., 2002; Zeke et al., 2005).
The first potential target of PP5 was identified by Chinkers, who demonstrated an interaction between PP5 and the atrial natriuretic peptide (ANP) receptor of the heart (Chinkers, 1994). Also in the heart, a decrease of PP5 expression has been observed in mice subjected to aldosterone treatment (Turchin et al., 2006). Curiously, another steroid, estradiol, up-regulates PP5 expression in MCF-7 breast cancer cells (Urban et al., 2001). Although the purpose of steroidal control over PP5 expression is not clear, it should be noted that estrogen and mineralocorticoid receptors are chaperoned by TPR-containing HSP90 complexes (Pratt and Toft, 1997), suggesting that feedback regulation may occur via PP5 or other TPR proteins.
There is considerable evidence for involvement of PP5 in several phosphorylation cascades. PP5 has been identified as a key effector for inactivation of MAPK signaling. Three major MAPK signal components affected by PP5 are Rac GTPase, Raf and ASK1. Additionally, PP5 appears to play a role in cell cycle progression in several ways. Treatment of cells with PP5 antisense RNA leads to hyperphosphorylation of p53 and subsequent G1 growth arrest (Chinkers, 2001). PP5 also binds to two proteins, CDC16 and CDC27, which are members of the anaphase-promoting complex (APC) – a complex required for anaphase initiation and the exit from mitosis (Chinkers, 2001). Lastly, it is now known that PP5 plays an important role in DNA-damage repair and cell cycle arrest by attenuating the activities of two closely-related checkpoint kinases, ATM (ataxia telangiectasia mutated) and ATR (ATM and Rad3 related). These early studies relied on PP5 knock-down by RNAi. More recent work utilizing cells from PP5-deficient mice have confirmed the role of PP5 in ATM signaling (Yong et al., 2007).
PP5 also regulates the activity of transcription factors. The cellular stress response is mediated by heat shock factor (HSF1), which acts by up-regulating expression of heat shock proteins, most notably HSP70 and HSP90. Curiously, HSF1 is maintained in the inactive state by association with HSP90 complexes containing TPRs, including PP5. A recent report suggests that PP5 is a negative regulator of HSF1 by preventing or reversing its active, hyperphosphorylated state (Conde et al., 2005).
Although it is widely speculated that PP5 interacts with most members of the steroid receptor family, direct evidence only exists for the estrogen (ER) and glucocorticoid receptors. In both cases, interaction with PP5 is mediated by HSP90. The first evidence for an ER/PP5 functional relationship was provided by the Honkanen laboratory, who showed estradiol stimulation of PP5 expression in MCF-7 breast cancer cells. In this system, PP5 promotes proliferation of breast cancers cells in culture (Urban et al., 2001) and growth of tumors in a mouse xenograph model (Golden et al., 2004). Using a yeast two-hybrid assay, Inoue and colleagues (Ikeda et al., 2004) identified PP5 as a binding partner of ER and localized the interaction to the ER ligand-binding domain – the known binding region of HSP90 (Pratt and Toft, 1997). By altering expression levels of PP5 it was shown that the phosphatase acts as a negative regulator of ER activity at several ER-responsive genes (Ikeda et al., 2004).
Even before PP5 was discovered, evidence for phosphatase regulation of GR had been reported. In cells treated with okadaic acid or related inhibitors a potentiation of GR activity at heterologous reporter genes was observed (Somers and DeFranco, 1992). Although these data suggest that PP5 is a negative regulator of GR, the broad-spectrum activity of okadaic acid on members of the PPP family made this only a tentative conclusion. More direct evidence was provided by Chinkers and Pratt, who demonstrated the presence of PP5 in GR complexes, as well as inhibition of GR transcriptional activity in the presence of a TPR dominant-negative peptide (Chen et al., 1996). This would suggest that PP5 is a positive regulator of GR. Yet, problems of interpretation also occur here, as the TPR peptide quite likely blocked interaction of other receptor-associated TPR proteins, such as FKBP52, FKBP51 or Cyp40. A more specific approach was provided by Honkanen and colleagues who used antisense RNA to down-regulate expression of PP5 (Zuo et al., 1999). Their results showed increased GR activity at heterologous reporters and increased susceptibility to hormonal growth arrest. Yet, a more recent study by Garabedian came to the opposite conclusion, as antisense knockdown of PP5 reduced GR activity at three endogenous genes (Wang et al., 2007). Although potential reasons for this discrepancy are many, it is interesting to note that PP5 knockdown did not reduce GR activity at a fourth endogenous gene (Wang et al., 2007), suggesting that PP5 somehow differentially controls GR transcriptional activity in a gene-specific fashion.
The above data suggests that PP5 is an important regulator of GR transcriptional activity. Several underlying mechanisms have been proposed to explain this phenomenon. First, PP5 may regulate the intrinsic ability of GR to bind glucocorticoids, as GR complexes containing PP5 have higher binding affinity for dexamethasone compared to complexes containing FKBP51 (Davies et al., 2005). Second, PP5 may control nuclear translocation of GR due to its ability to interact with the motor protein dynein via its PPIase-like domain (Galigniana et al., 2002) (Fig. 1). Lastly, PP5 may affect the ability of GR to interact with transcriptional co-regulators based on its phosphorylation state (Wang et al., 2007). In response to hormone, GR phosphorylation at serines 203, 211 and 226 is usually observed. Interestingly, PP5 can dephosphorylate GR at each of these sites, but the most pronounced effect was at S226, which is the primary site for inactivation of GR via the c-Jun N-terminal kinase (JNK) signal pathway. In light of these findings, we propose the working model seen in Fig. 2 in which the primary role of PP5 is to reset the basal phosphorylation state of GR at specific phospho-residues. It is likely that PP5 performs this function during de novo synthesis of GR (not shown) and for GR that is recycling to the cytoplasm after release from chromatin.
Fig. 2.
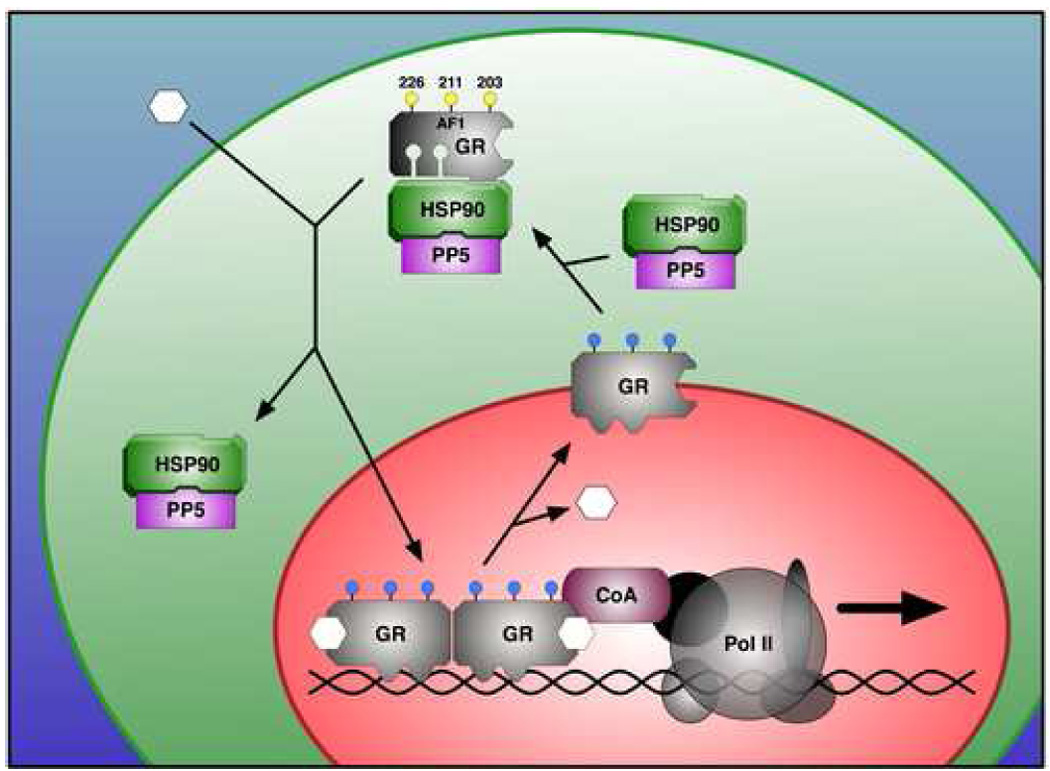
Model of PP5 actions on glucocorticoid receptor (GR). In most cells at least a fraction of GR/HSP90 heterocomplexes contain PP5 as the TPR protein component. In response to hormone, several well-documented events take place: 1) dissociation of GR from the heterocomplex, 2) dimerization of GR and binding to chromatin at gene promoters, and 3) hyperphosphorylation of GR, particularly residues S203, S211 and S226 (phopho-serines are in blue) of the AF-1 domain. It is now known that phosphorylation is required for maximal GR transciptional activity and that PP5 contributes to GR dephosphorylation at the indicated residues. We therefore propose that the main function of PP5 is to reset the phosphorylation state of GR, most likely during GR recycling to the cytoplasm and at de novo synthesis (not shown). It is also possible that GR hyperphosphorylation may increase as a consequence of PP5 release, in addition to active phosphorylation by kinases. PP5 may also contribute to GR action by altering its ability to bind hormone and to translocate to the nucleus (not shown).
As we move forward, the issue of mechanism will be clarified. Meanwhile, it is already clear that PP5 is a ubiquitous master modulator with pleiotropic effects on cellular functions. Because PP5 has pro-proliferative properties in most cells, it is a promising therapeutic target in the treatment of cancer. Successful development of such drugs may require targeting of the phosphatase domain with derivatives of okadaic acid, or FK506-related compounds that bind the PPIase-like domain, or the targeting of the TPR domain to perhaps block a subset of PP5 actions requiring TPR clients.
Acknowledgements
This work was supported in part by a National Institutes of Health (USA) grant (DK70127) to E.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker W, Kentrup H, Klumpp S, Schultz JE, Joost HG. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J Biol Chem. 1994;269:22586–22592. [PubMed] [Google Scholar]
- Chen MS, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. doi: 10.1074/jbc.271.50.32315. [DOI] [PubMed] [Google Scholar]
- Chen MX, Cohen PT. Activation of protein phosphatase 5 by limited proteolysis or the binding of polyunsaturated fatty acids to the TPR domain. FEBS Lett. 1997;400:136–140. doi: 10.1016/s0014-5793(96)01427-5. [DOI] [PubMed] [Google Scholar]
- Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PT. A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J. 1994;13:4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M. Targeting of a distinctive protein-serine phosphatase to the protein kinase-like domain of the atrial natriuretic peptide receptor. Proc Natl Acad Sci U S A. 1994;91:11075–11079. doi: 10.1073/pnas.91.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M. Protein phosphatase 5 in signal transduction. Trends Endocrinol Metab. 2001;12:28–32. doi: 10.1016/s1043-2760(00)00335-0. [DOI] [PubMed] [Google Scholar]
- Conde R, Xavier J, McLoughlin C, Chinkers M, Ovsenek N. Protein phosphatase 5 is a negative modulator of heat shock factor 1. J Biol Chem. 2005;280:28989–28996. doi: 10.1074/jbc.M503594200. [DOI] [PubMed] [Google Scholar]
- Das AK, Cohen PW, Barford D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein-protein interactions. EMBO J. 1998;17:1192–1199. doi: 10.1093/emboj/17.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TH, Ning YM, Sanchez ER. Differential Control of Glucocorticoid Receptor Hormone-Binding Function by Tetratricopeptide Repeat (TPR) Proteins and the Immunosuppressive Ligand FK506. Biochemistry. 2005;44:2030–2038. doi: 10.1021/bi048503v. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Harrell JM, Murphy PJ, Chinkers M, Radanyi C, Renoir JM, Zhang M, Pratt WB. Binding of hsp90-associated immunophilins to cytoplasmic dynein: direct binding and in vivo evidence that the peptidylprolyl isomerase domain is a dynein interaction domain. Biochemistry. 2002;41:13602–13610. doi: 10.1021/bi020399z. [DOI] [PubMed] [Google Scholar]
- Golden T, Aragon IV, Zhou G, Cooper SR, Dean NM, Honkanen RE. Constitutive over expression of serine/threonine protein phosphatase 5 (PP5) augments estrogen-dependent tumor growth in mice. Cancer Lett. 2004;215:95–100. doi: 10.1016/j.canlet.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ogawa S, Tsukui T, Horie-Inoue K, Ouchi Y, Kato S, Muramatsu M, Inoue S. Protein phosphatase 5 is a negative regulator of estrogen receptor-mediated transcription. Mol Endocrinol. 2004;18:1131–1143. doi: 10.1210/me.2003-0308. [DOI] [PubMed] [Google Scholar]
- Kang H, Sayner SL, Gross KL, Russell LC, Chinkers M. Identification of amino acids in the tetratricopeptide repeat and C-terminal domains of protein phosphatase 5 involved in autoinhibition and lipid activation. Biochemistry. 2001;40:10485–40490. doi: 10.1021/bi010999i. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18(3):306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Russell LC, Whitt SR, Chen MS, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem. 1999;274:20060–20063. doi: 10.1074/jbc.274.29.20060. [DOI] [PubMed] [Google Scholar]
- Skinner J, Sinclair C, Romeo C, Armstrong D, Charbonneau H, Rossie S. Purification of a fatty acid-stimulated protein-serine/threonine phosphatase from bovine brain and its identification as a homolog of protein phosphatase 5. J Biol Chem. 1997;272:22464–22471. doi: 10.1074/jbc.272.36.22464. [DOI] [PubMed] [Google Scholar]
- Somers JP, DeFranco DB. Effects of okadaic acid, a protein phosphatase inhibitor, on glucocorticoid receptor-mediated enhancement. Mol Endocrinol. 1992;6:26–34. doi: 10.1210/mend.6.1.1310797. [DOI] [PubMed] [Google Scholar]
- Swingle MR, Honkanen RE, Ciszak EM. Structural basis for the catalytic activity of human serine/threonine protein phosphatase-5. J Biol Chem. 2004;279:33992–33999. doi: 10.1074/jbc.M402855200. [DOI] [PubMed] [Google Scholar]
- Turchin A, Guo CZ, Adler GK, Ricchiuti V, Kohane IS, Williams GH. Effect of Acute Aldosterone Administration on Gene Expression Profile in the Heart. Endocrinology. 2006 doi: 10.1210/en.2005-1674. [DOI] [PubMed] [Google Scholar]
- Urban G, Golden T, Aragon IV, Scammell JG, Dean NM, Honkanen RE. Identification of an estrogen-inducible phosphatase (PP5) that converts MCF-7 human breast carcinoma cells into an estrogen-independent phenotype when expressed constitutively. J Biol Chem. 2001;276:27638–27646. doi: 10.1074/jbc.M103512200. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chen W, Kono E, Dang T, Garabedian MJ. Modulation of glucocorticoid receptor phosphorylation and transcriptional activity by a C-terminal-associated protein phosphatase. Mol Endocrinol. 2007;21:625–634. doi: 10.1210/me.2005-0338. [DOI] [PubMed] [Google Scholar]
- Yong W, Bao S, Chen H, Li D, Sanchez ER, Shou W. Mice lacking protein phosphatase 5 are defective in ataxia telangiectasia mutated (ATM)-mediated cell cycle arrest. J Biol Chem. 2007;282:14690–14694. doi: 10.1074/jbc.C700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeke T, Morrice N, Vazquez-Martin C, Cohen PT. Human protein phosphatase 5 dissociates from heat-shock proteins and is proteolytically activated in response to arachidonic acid and the microtubule-depolymerizing drug nocodazole. Biochem J. 2005;385:45–56. doi: 10.1042/BJ20040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–8857. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]


