Abstract
OBJECTIVE:
Plateau in testicular cancer incidence in some parts of the United States (US) especially among non-Hispanic white males in Los Angeles had been observed. We conducted three decades temporal trends analysis to assess the evidence of such a plateau, and to examine whether the rate remains stable across racial/ethnic groups as well as the influence of age at diagnosis on the incidence rate.
STUDY DESIGN:
Population-based temporal trends analysis.
METHODS:
Using the Surveillance Epidemiology and End Results (SEER), we identified between 1975 and 2004, 16,580 of newly diagnosed testicular cancer cases, aged 15−49 years. The incidence rates were examined by calculating the age-adjusted rates and their 95% Confidence Interval (CI) for the age at diagnosis, SEER areas, and race by the year of diagnosis. The percent change and annual percent change were examined for trends.
RESULTS:
Incidence of testicular cancer continues to increase among US males, albeit the plateau of the 1990s. Between 1975 and 2004 the age-adjusted incidence rate for ages, 15−49 years increased from 2.9 (1975) to 5.1(2004) per 100,000. The trends indicated a percent change of 71.9% and a statistically significant annual percent change of 1.6 %,( 95% CI, 1.3−2.0), p < 0.05. Though the rates in blacks remained strikingly low, 0.3 to 1.4 per 100,000, the highest annual percent change was observed among blacks, 2.3%, (95%, CI, 0.8−3.9), p < 0.05 for trends. The rates were intermediate among Asians/ Pacific Islanders and American Indian and Alaska Natives 0.7 to 2.9 per 100,000, percent change (117.3%) and a statistically significant annual change of 1.5%, (95% CI, 0.3−2.7) p < 0.05 for trends. The highest rates were reported among Whites, 3.2 to 6.3 per 100,000, percent change (90.4%) , with a statistically significant annual percent change of 2.0%, (95% CI, 1.6−2.3), p < 0.05. The peak age at diagnosis was, 30−34 years while the lowest rates were reported in 15−19 age group. Likewise, incidence rates varied by SEER areas with predominantly white states representing areas associated the highest reported rates.
CONCLUSIONS:
Overall, testicular cancer incidence rate remains to plateau in the United States, while racial variance persists in rates, black males demonstrated the highest increase in the annual percent change. Further studies are needed to examine the recent increase among black males and the potential determinants.
Keywords: testicular cancer, incidence rates, race, population-based study, trends
INTRODUCTION
Testicular cancer is the most diagnosed malignancy among young men age 15 to 34 years.1 Testicular neoplasms are primarily Germ cell tumors (GCTs), which are a morphologically distinct group of neoplasms with varied clinical presentation. Ninety-five percent of tumors arising in the testes are GCTs, indicating a primordial germ cell origin.2-3 Testicular cancers are histopathologically classified mainly as seminomas or nonseminomas, with the latter being predominantly teratomas.4 Whereas these two types of tumors have different clinical features and possibly different etiologies, they are considered together in trends studies. In addition, temporal trends for the two major histologic types of testicular cancer have been observed to be similar, suggesting common etiologic factors.5 Since temporal trends studies are primarily aimed at generating and testing hypotheses on candidate exposures responsible for increasing or decreasing incidence rather than at assessing etiologic heterogeneities, this trends study evaluates seminomas and non-seminomas together unless otherwise specified.
Geographic variation had been observed in testicular incidence rates. In fact, the incidence of testicular germ cell tumors has been highest among whites in the United States since 1940, 6-7 and has been steadily rising in the past forty to fifty years in western countries, especially in populations with European ancestry.8 Moreover, the 1993−1997 international age-adjusted incidence rates varied ten-fold across populations, with the highest rate in Denmark (9.9 per 100,000) and the lowest in Zimbabwe (0.4 per 100,000).9 Comparatively, incidence is lowest in Asian and African countries, intermediate in the United States and highest in the Scandinavian countries. 10,11,12
In the United States, a geographic pattern has been observed in the SEER registries. Rates are higher in registries with predominately white populations and lower in registries with ethnic minorities. Urban and rural differences in testicular cancer have been studied with varying results. 13-15 The clustering of testicular cancer in these SEER registries may result from shared environment, shared genes (biology), both, or may be associated with random factors.
Regarding age, the distribution of testicular cancer is remarkably distinct. Testicular cancer rarely occur before puberty, arises throughout adolescent, peaks at age 25 to 29 years and declines gradually thereafter.5 Germ cell carcinogenesis is thought to arise in the population of primordial germ cells, evolves through the stages of carcinoma in situ, and finally progresses to invasive cancer after the onset of puberty, most often in the twenties and thirties.3 This pattern of age-at-incidence distribution of testicular cancer differs from most malignancies, which normally peak much later in life and increases with advancing age.
With respect to race, the incidence of testicular cancer varies overall, with extremely low rates observed in blacks and other non-white races in comparison to Caucasian populations.16 In the United States, there is significant variation in testicular neoplasm incidence. The rate is intermediate in Asians,17 lowest in Blacks and highest in White Americans. Testicular cancer thus presents a rare incidence rate for cancer by racial distribution, comparing black versus white cancer rates in United States. This observation remains one of the most intriguing perspectives of testicular cancer epidemiology in the United States. Therefore, this racial variation may be due to shared environment, biology (shared genes), personal behavior, life style pattern, exposure to different environment and/or occupational exposure differences.
Despite the above, the incidence of testicular neoplasm has been observed in recent findings to plateau in some parts of the United States18. To examine this hypothesis, we conducted a trends study to examine the pattern and incidence of testicular cancer from 1975 through 2004 for evidence or lack of evidence for such plateau and to explore possible explanatory factors on exposures whose prevalence may have decreased or increased over the past few years, which are common enough to influence the incidence rate. To our knowledge, very few studies on testicular cancer have been performed examining the geographic locale incidence rate and furthermore, even fewer have attempted to account for the racial disparity in testicular tumors. Very interestingly, none has covered three decades (1975−2004) in the incidence trends analyses. Therefore, given the limited knowledge of the well established etiologic risk factors, this present trends study provides the opportunity for hypothesis testing on factors that may explain the increasing trends, the distinctive age at diagnosis of testicular tumors, geographic variation, and the racial disparities in testicular cancer incidence rate.
MATERIALS AND METHODS
Data Source
We obtained the incidence rates data for testicular cancer from 1975 through 2004 for cases by 5-year age intervals at age 15 through 49 years. The Surveillance Epidemiology End Results database is the National Cancer Institute (NCI) 19 tumor registry for epidemiologic (cancer incidence, mortality and survivability, etc.) and health research, such as diagnosis and treatment received. This database comprises population-based tumor registries that routinely collect information on all newly diagnosed cancer cases that occur in persons residing in the SEER areas or registry, termed geographic locale. The database includes patients' socio-demographic information (ethnicity/race, month, day and year of birth, place of birth, residence, marital status), age at diagnosis, tumor stage and grade, tumor type (primary tumor and histologic types), tumor markers, surgery, radiation received, vital status (follow-up – dead or alive) and cause-specific mortality.
Data Collection
The SEER data collection commenced in January 1, 1973,19 with the primary SEER areas, namely the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii, as well as the metropolitan areas of Detroit, Michigan, San Francisco/Oakland, California. In 1974 and 1975, the Seattle-Puget Sound, Washington and Atlanta metropolitan joined the SERR registry respectively. These areas represent an estimated 11% of the US population. In 1992, Los Angeles County and San Jose-Monterey area joined the SEER registry for racial minorities' representation. The SEER areas comprise the metropolitan areas of San Francisco/Oakland, Detroit, Atlanta, and Seattle; Los Angeles county, the San Jose-Monterey area; and the states of Connecticut, Iowa, New Mexico, Utah, and Hawaii.19 These areas represent an estimated 14% of the United States population. In 2000, the States of Kentucky, Louisiana, New Jersey and the other areas of California, which were not previously included in the two phases of the SEER registry, joined the registry. Overall, the present SEER registry comprises 17 registries.
We utilized the SEER data for the period 1975 to 2004, from the nine SEER registries which covers an estimated 11% of the US population. SEER data on testicular cancer are available from 1973. However, the 1975−2004 represents the only years for which all nine registries have cases in the database. This data is considered highly valid and representative of the United States population. These registries are recognized by the North American Association of Cancer Registries for the highest level of certification of data quality .20
Study Population
The study population comprised men with newly diagnosed testicular cancer between 1992 and 2002, age 15 to 49 years in the 9 SEER registries. All races were included in the study that met the inclusion criteria of age at diagnosis. Between 1975 and 2004, 16,580 cases of testicular cancer were diagnosed among men age 15 to 49 years in the nine SEER registries. This comprised whites, 15,464 (93.3%), blacks, 353 (2.1%), American Indian/Alaska Natives and Asian or Pacific Islanders, 646 (3.9%) and other unspecified,117 (0.7%).
Statistical Analysis
The nine oldest SEER area registry data from 1975 to 2004 was used to calculate the age-adjusted rates for the geographic locale, race and age at diagnosis (15−49) years. Rates, standard errors and 95% Confidence Intervals for the rates were calculated. The rates were per 100,000 and age-adjusted to the 2000 US standard population. Percent change was calculated using 1 year for each end point, while the annual percent change (APC) was calculated using weighted least squares method. The APC is the average rate of change in a rate over several years and is used to measure trends over time (1975−2004). Statistical significance (meaning, APC is significantly different from zero) was assessed, utilizing p <0.05 for APC and 95% confidence interval (CI) for rates and trends. All statistical analyses were performed using the most recent SEER statistical package, SEER*Stat 6.3.5.
RESULTS
There were 16,580 reported cases of testicular cancer among US males aged 15−49 years between January 1, 1975 and December 31, 2004 in the nine oldest SEER registries, which represent an estimated 11% of the total US population. The three decades (1975−2004) testicular incidence trends demonstrated 71.9 percent change, with a statistically significant annual change of 1.6% (95% CI, 1.3−2.0), p < 0.05. The trends for the selected decades, 1975−1984, 1985−1994 and 1995−2004 were 27.7%, 24.4% and 27.9% percent change respectively, with 3.1% (95% CI, 1.3−4.8),1.7%(95% CI, 0.6−2.8) and 1.6% (95% CI, 0.0−3.2) as the corresponding annual percent change, and were statistically significantly different from zero, p < 0.05 .
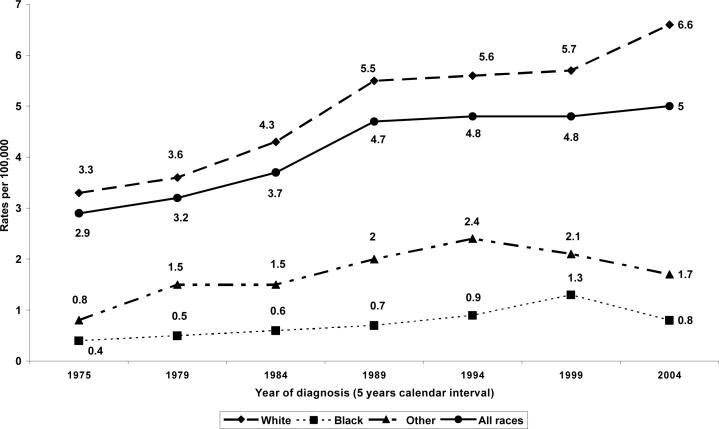
Table 1 presents the race non-specific (total males population as all races) and race-specific age-adjusted testicular incidence rates among the US males, from 1975 to 2004. The race non-specific age-adjusted average annual rates for the indicated period of time (5 years calendar interval) increased steadily among US males, from 3.1 per 100,000 (1975−1979) to 4.9 per 100,00 (200−2004). During the three decades, there was a 90.4% change and a statistically significant 2.0% annual percent change in rates for white, p < 0.05. The rates were remarkably higher among US white males, with steady increase through the late 1980s (3.5 per 100,000 in 1975−1979 to 4.9 per 100,000 in 1985−1989), stabilizing in the second half of the 1990s (5.4 per 100,000 in 1990−1994 to 5.5 per 100,000 in 1995−1999), followed by moderate increase in the 2000s (6.1 per 100,000) (Table 1). While the rates were relatively low among blacks (0.3 in 1997 to 1.3 per 100,000 in 1998) the percent change was higher than that of the total US population, 77.7%, and the annual percent change for trends was statistically significant and highest, compared to other racial/ethnic groups and the total US population, 2.3%, (95% CI, 0.8−3.9), p < 0.0.5.(data not shown )
TABLE 1.
Race and age-adjusted incidence rates of testicular cancer (per 100,000) for US males (15−49 years) from 1975−2004: Surveillance, Epidemiology and End Results Program
| Years | Race (age-adjusted incidence rate & 95% CI) | Total US Population | ||
|---|---|---|---|---|
| White | Black | Other | ||
| 1975 | 3.3 (2.9−3.8) | 0.4 (0.1−1.1) | 0.8 (0.2−1.9) | 2.9 (2.6−3.3) |
| 1976 | 3.2 (2.8−3.6) | 0.9 (0.3−1.8) | 1.4 (0.6−2.8) | 2.9 (2.5−3.2) |
| 1977 | 4.1 (3.7−4.5) (3 .5)* | 0.3 (0.1−0.9) (0.7) | 0.7 (0.2−1.7) (1.1) | 3.6 (3.2−4.0) (3.1) |
| 1978 | 3.3 (2.9−3.7) | 1.4 (0.7−2.4) | 1.1 (0.4−2.2) | 3.0 (2.6−3.3) |
| 1979 | 3.6 (3.2−4.0) | 0.5 (0.2−1.1) | 1.5 (0.7−2.9) | 3.2 (2.8−3.5) |
| 1980 | 4.2 (3.8−4.7) | 0.6 (0.3−1.4) | 1.4 (0.7−2.6) | 3.7 (3.4−4.1) |
| 1981 | 4.0 (3.6−4.5) | 0.6 (0.2−1.2) | 1.9 (1.1−3.2) | 3.5 (3.9−4.0) |
| 1982 | 4.2 (3.8−4.6) (4.2) | 0.5 (0.2−1.0) (0.6) | 1.2 (0.6−2.1) (1.4) | 3.6 (3.3−4.0) (3.7) |
| 1983 | 4.5 (4.1−5.0) | 0.7 (0.3−1.4) | 1.2 (0.6−2.2) | 3.9 (3.5−4.3) |
| 1984 | 4.3 (3.9−4.8) | 0.6 (0.2−1.2) | 1.5 (0.8−2.5) | 3.7 (3.4−4.1) |
| 1985 | 4.5 (4.1−4.9) | 0.9 (0.5−1.6) | 1.1(0.5−2.1) | 3.8 (3.5−4.2) |
| 1986 | 4.8 (4.4−5.3) | 0.4 (0.1−0.9) | 1.6 (0.9−2.6) | 4.1 (3.7−4.5) |
| 1987 | 5.0 (4.6−5.5) (4.9) | 0.9 (0.4−1.6) (0.7) | 2.4 (1.5−3.5) (1.7) | 4.4 (4.0−4.8) (4.2) |
| 1988 | 4.9 (4.5−5.3) | 0.5 (0.2−1.0) | 1.2 (0.6−2.1) | 4.1 (3.7−4.5) |
| 1989 | 5.5 (5.1−6.0) | 0.7 (0.3−1.3) | 2.0 (1.3−3.0) | 4.7 (4.3−5.1) |
| 1990 | 5.3 (4.9−5.8) | 0.3 (0.1−0.8) | 1.8 (1.2−2.8) | 4.4 (4.1−4.8) |
| 1991 | 5.0 (4.6−5.4) | 0.7 (0.3−1.3) | 2.9 (2.0−4.0) | 4.4 (4.0−4.7) |
| 1992 | 5.5 (5.0−5.9) (5.4) | 0.5(0.2−1.0) (0.6) | 1.5 (0.9−2.3) (2.1) | 4.5 (4.2−4.9) (4.5) |
| 1993 | 5.4 (4.9−5.8) | 0.6 (0.3−1.1) | 1.8 (1.2−2.7) | 4.5 (4.1−4.9) |
| 1994 | 5.6 (5.2−6.1) | 0.9 (0.5−1.6) | 2.4 (1.6−3.4) | 4.8 (4.4−5.1) |
| 1995 | 4.6 (4.2−5.0) | 1.3 (0.8−1.9) | 1.9 (1.3−2.8) | 3.9 (3.6−4.3) |
| 1996 | 5.6 (5.1−6.0) | 0.7 (0.3−1.2) | 1.7 (1.1−2.5) | 4.6 (4.3−5.0) |
| 1997 | 5.5 (5.1−6.0) (5.5) | 0.8 (0.4−1.4) (1.1) | 1.9 (1.3−2.8) (1.8) | 4.6 (4.2−4.9) (4.6) |
| 1998 | 6.1 (5.6−6.6) | 1.3 (0.8−1.9) | 1.6 (1.0−2.3) | 5.0 (4.7−5.4) |
| 1999 | 5.7 (5.3−6.2) | 1.3 (0.8−2.0) | 2.1 (1.5−3.0) | 4.8 (4.4−5.2) |
| 2000 | 6.1 (5.6−6.6) | 1.3 (0.8−1.9) | 2.4 (1.7−3.3) | 5.1 (4.7−5.5) |
| 2001 | 5.8 (5.4−6.3) | 1.2 (0.7−1.8) | 2.2 (1.5−3.0) | 4.8 (4.5−5.2) |
| 2002 | 6.3 (5.8−6.8 (6.1) | 1.0 (0.6−1.6) (1.0) | 2.0 (1.4−2.8) (2.0) | 5.1 (4.7−5.5) (4.9) |
| 2003 | 5.8 (5.3−6.3) | 0.8 (0.5−1.4) | 1.7 (1.1−2.4) | 4.7 (4.3−5.0) |
| 2004 | 6.3 (5.9−6.8) | 0.8 (0.4−1.3) | 1.7 (1.1−2.4) | 5.0 (4.7−5.4) |
Average of the annual rates for the indicated period of time.
Figure 1 displays steady increase rates and positive linear trends for three decades of age-adjusted testicular cancer incidence by five year calendar interval. In the total US population (race non-specific), testicular cancer rate increased steadily from 2.9 per 100,00 in 1975 to 3.7 per 100,00 in 1984, and sharply increased to 4.7 per 100,000 in 1989, then plateau, 4.8 per 100,000 in 1994 and 1999 (4.8 per 100,00), and peaked by 2004 (5.0 per 100,000), with a strong positive linear trends, R2=0.88. A similar pattern was observed among US white males, with a higher peak incidence rates in 2004 (6.6 per 100,000) and a stronger positive linear trend, R2=0.94. The rates increased steadily, though slowly among blacks, with the peak incidence rate in 1999 (1.3 per 100,000) and a moderate positive linear trends, R2=0.64.
Figure 1.
Three decades (1975−2004) testicular cancer incidence rates among US males, aged 15−49 years by race/ethnicity
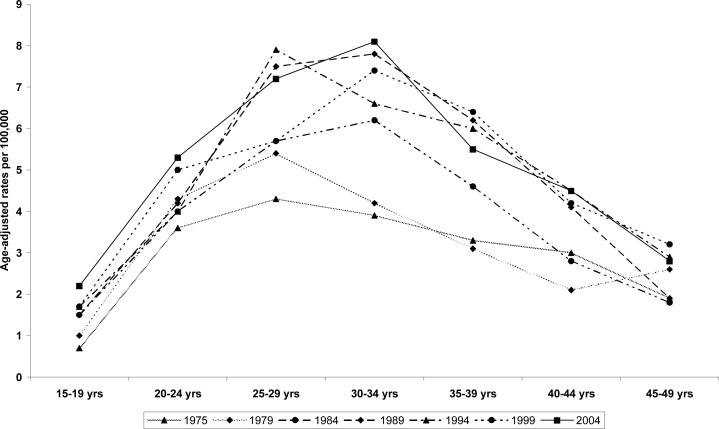
Table 2 presents the age-specific, age-adjusted incidence of testicular cancer rate (race non-specific and race-specific) during 1975 through 2004. Regardless of the age at diagnosis, incidence rates were lowest in the second half of the 1970s (1.1, 3.7, 3.9, 3.5, 2.7 and 2.2 per 100,00) for age groups,(15−19, 20−24, 30−34, 35−39, 40−44 and 45−49) years respectively; and highest in the first half of the 2000s (1.8, 5.1, 6.4, 7.0, 6.1, 5.1 and 3.1 per 100,000) for age groups, (15−19, 20−24, 25−29, 30−34, 35−39, 40−44 and 45−49 years) respectively. Irrespective of the calendar year of diagnosis, the incidence rate was lowest among age group 15−19 years and peaked at age group, 30−34 years (whites and all races).
TABLE 2.
Age-specific (15−49 years) and age-adjusted incidence rates of testicular cancer (per 100,000) for US males from 1975−2004: Surveillance, Epidemiology and End Results Program
| Year | Age (years) | ||||||
|---|---|---|---|---|---|---|---|
| 15−19 | 20−24 | 25−29 | 30−34 | 35−39 | 40−44 | 45−49 | |
| 1975 | 0.7 | 3.6 | 4.3 | 3.9 | 3.3 | 3.0 | 1.9 |
| 1976 | 1.4 | 3.7 | 4.8 | 3.0 | 3.0 | 2.0 | 2.6 |
| 1977 | 1.1(1.1)* | 3.3(3.7) | 4.8(4.8) | 4.8(3.9) | 4.6(3.5) | 4.2(2.7) | 2.1(2.2) |
| 1978 | 1.2 | 3.4 | 4.8 | 3.6 | 3.7 | 2.3 | 1.9 |
| 1979 | 1.0 | 4.3 | 5.4 | 4.2 | 3.1 | 2.1 | 2.6 |
| 1980 | 1.9 | 4.4 | 5.2 | 5.8 | 3.4 | 3.1 | 2.4 |
| 1981 | 1.3 | 3.5 | 5.2 | 5.8 | 4.2 | 2.8 | 2.1 |
| 1982 | 1.5(1.6) | 4.4(4.1) | 5.6(5.5) | 4.6(5.5) | 3.5(4.1) | 3.5(3.0) | 2.5(2.3) |
| 1983 | 1.8 | 4.3 | 6.0 | 5.0 | 4.6 | 3.0 | 2.8 |
| 1984 | 1.5 | 4.0 | 5.7 | 6.2 | 4.6 | 2.8 | 1.8 |
| 1985 | 1.1 | 5.4 | 5.9 | 5.3 | 4.5 | 2.8 | 2.2 |
| 1986 | 1.6 | 4.3 | 5.7 | 6.1 | 5.0 | 3.7 | 2.4 |
| 1987 | 1.1(1.3) | 4.3(4.5) | 6.6(6.3) | 7.1(6.2) | 5.9(5.5) | 3.7(3.7) | 2.2(2.3) |
| 1988 | 1.2 | 4.2 | 5.8 | 5.6 | 5.8 | 3.5 | 2.6 |
| 1989 | 1.5 | 4.2 | 7.5 | 6.9 | 6.2 | 4.7 | 1.9 |
| 1990 | 1.0 | 5.2 | 6.0 | 7.8 | 5.0 | 4.1 | 2.3 |
| 1991 | 2.0 | 3.7 | 6.7 | 6.6 | 4.9 | 4.1 | 2.9 |
| 1992 | 1.9(1.6) | 3.8(4.2) | 5.9(6.5) | 7.2(7.0) | 5.5(5.4) | 4.4(4.2) | 3.1(2.8) |
| 1993 | 1.5 | 4.3 | 6.1 | 7.0 | 5.8 | 4.1 | 2.7 |
| 1994 | 1.7 | 4.0 | 7.9 | 6.6 | 6.0 | 4.5 | 2.9 |
| 1995 | 1.7 | 3.5 | 6.3 | 5.9 | 5.4 | 3.1 | 1.9 |
| 1996 | 1.3 | 3.8 | 6.5 | 7.3 | 6.1 | 4.5 | 2.8 |
| 1997 | 1.6(1.6) | 3.8(4.0) | 5.9(6.3) | 7.5(6.9) | 6.1(6.1) | 4.0(4.3) | 3.2(3.0) |
| 1998 | 1.5 | 4.1 | 7.0 | 6.5 | 6.4 | 5.7 | 3.9 |
| 1999 | 1.7 | 5.0 | 5.7 | 7.4 | 6.4 | 4.2 | 3.2 |
| 2000 | 1.7 | 5.4 | 5.8 | 6.8 | 6.5 | 5.8 | 3.5 |
| 2001 | 1.9 | 5.6 | 6.2 | 6.3 | 5.2 | 5.7 | 3.0 |
| 2002 | 1.9(1.8) | 4.6(5.1) | 6.3(6.4) | 7.3(7.0) | 7.4(6.1) | 5.2(5.1) | 2.8(3.1) |
| 2003 | 1.4 | 4.8 | 6.5 | 6.4 | 6.1 | 4.2 | 3.5 |
| 2004 | 2.2 | 5.3 | 7.2 | 8.1 | 5.5 | 4.5 | 2.8 |
Average of annual rates for the indicated period of time
In the race non-specific and white males populations, there was a steady increase in the incidence rate in the selected years (1975, 1979, 1984, 1989, 1994, 1999, and 2004) of reported cases regardless of the age at diagnosis (Figure 2). In this sample (race non-specific), the peak age at diagnosis was 30−34 years in the total US males population (8.1 per 100,000 in 2004) as well as among the white males (10.5 per 100,000 in 2004). Overall, incidence rates were lowest in the age group,15−19 years (0.7 per 100,000 in 1975 and 2.0 per 100,00 in 2004) , peaked at 30−34 years ( 3.9 per 100,000 in 1975 and 8.1 per 100,000 in 2004) and declined in the age-group, 45−49 years ( 1.9 per 100,000 in 1975 and 2.8 per 100,000 in 2004). Among the Asian /Pacific Islanders & American Indian/Alaska native, the peak age at diagnosis was the age group, 25−29 years (2.0 per 100,000 in 1975 and 3.2 per 100,000 in 2004) compared with age group 15−19 years (0.0 per 100,000 in 1975 and 0.4 per 100.000 in 2004) and age group, 45−49 years (0.0 per 100.000 in 1975 and 1.1 per 100,000 in 2004).(Data not shown).
Figure 2.
Three decades (1975−2004) testicular cancer incidence rates among US males (15−49 years) by age at diagnosis
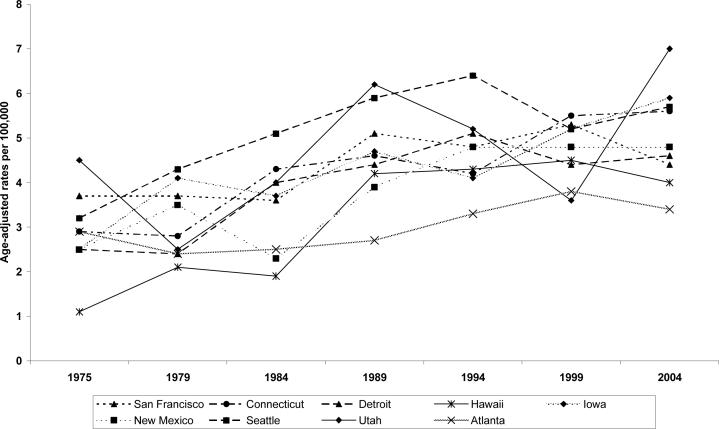
Table 3 presents the age-adjusted testicular cancer rate per 100,000 for the nine SEER areas/ registry or geographic locale, and age-specific incidence rates. The rates were highest in Utah, Iowa, Seattle and Connecticut, intermediate in New Mexico, San Francisco-Oakland and lowest in Atlanta, Detroit and Hawaii. In San Francis-Oakland, the incidence rates increased in the first half of the 1980s (3.3 per 100,000 in 1975−1979) to (4.0 per 100,000 in 1980−1984) and stabilized throughout the 1990s (4.6 per 100,000). Figure 3 displays the age-adjusted incidence rates by SEER areas in the selected years of diagnosis (1975, 1979, 1984, 1989, 1994, 1999 and 2004). In almost all SEER areas, the lowest incidence rates were reported in 1975 whereas the highest rates were in 2004.
TABLE 3.
Age-adjusted testicular cancer incidence rates in US males, ages 15−49 years by SEER Area, 1975−2004
| Year | Surveillance, Epidemiology and End Results Registries | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SF-O | Connec | Detriot | Hawaii | Iowa | NM | Seattle | Utah | Atlanta | |
| 1975 | 3.7 | 2.9 | 2.5 | 1.1 | 2.5 | 2.5 | 3.2 | 4.5 | 2.9 |
| 1976 | 2.8 | 3.5 | 2.5 | 3.1 | 3.1 | 1.9 | 2.8 | 3.2 | 2.6 |
| 1977 | 3.4(3.3)* | 3.8(3.1) | 3.2(2.7) | 3.3(2.4) | 2.8(3.1) | 3.9(3.0) | 4.8(3.7) | 4.1(3.8) | 2.8(2.1) |
| 1978 | 3.0 | 2.6 | 2.8 | 2.3 | 3.1 | 3.0 | 3.3 | 4.6 | 2.4 |
| 1979 | 3.7 | 2.8 | 2.4 | 2.1 | 4.1 | 3.5 | 4.3 | 2.5 | 2.4 |
| 1980 | 4.0 | 4.6 | 3.8 | 2.1 | 3.1 | 2.8 | 4.5 | 3.8 | 2.6 |
| 1981 | 4.5 | 3.8 | 3.3 | 3.0 | 2.9 | 2.9 | 3.9 | 3.9 | 2.8 |
| 1982 | 3.7(4.0) | 3.3(3.4) | 3.2(3.5) | 3.5(2.7) | 4.1(3.7) | 3.9(3.0) | 4.8(4.7) | 3.4(3.7) | 2.3(2.6) |
| 1983 | 4.3 | 4.3 | 3.3 | 2.8 | 4.5 | 3.2 | 5.0 | 3.2 | 2.6 |
| 1984 | 3.6 | 4.3 | 4.0 | 1.9 | 3.7 | 2.3 | 5.1 | 4.0 | 2.5 |
| 1985 | 3.8 | 3.5 | 3.6 | 3.2 | 3.3 | 3.5 | 5.2 | 4.6 | 3.7 |
| 1986 | 4.3 | 4.3 | 3.3 | 2.2 | 4.6 | 2.2 | 6.5 | 5.0 | 2.5 |
| 1987 | 5.0(4.6) | 4.6(4.1) | 4.1(3.9) | 4.6(3.4) | 4.6(4.3) | 3.6(3.4) | 5.4(5.5) | 4.4(4.8) | 2.3(3.0) |
| 1988 | 5.0 | 3.4 | 4.0 | 3.0 | 4.1 | 3.6 | 4.4 | 3.8 | 4.0 |
| 1989 | 5.1 | 4.6 | 4.4 | 4.2 | 4.7 | 3.9 | 5.9 | 6.2 | 2.7 |
| 1990 | 5.1 | 4.2 | 4.4 | 2.9 | 3.5 | 4.8 | 5.8 | 4.6 | 3.0 |
| 1991 | 4.7 | 5.3 | 3.9 | 5.2 | 3.7 | 4.6 | 4.6 | 4.3 | 3.2 |
| 1992 | 4.5(4.6) | 5.1(4.7) | 3.9(4.2) | 3.4(4.0) | 5.1(4.3) | 4.6(4.7) | 5.3(5.6) | 6.0(5.2) | 2.8(3.1) |
| 1993 | 3.9 | 4.8 | 3.9 | 4.0 | 5.1 | 4.8 | 5.7 | 5.8 | 3.0 |
| 1994 | 4.8 | 4.2 | 5.1 | 4.3 | 4.1 | 4.8 | 6.4 | 5.2 | 3.3 |
| 1995 | 4.3 | 4.0 | 3.4 | 4.9 | 4.3 | 3.0 | 4.9 | 4.0 | 2.7 |
| 1996 | 4.2 | 4.7 | 4.8 | 4.7 | 5.4 | 5.1 | 5.5 | 5.2 | 2.4 |
| 1997 | 4.1(4.6) | 4.8(5.0) | 4.4(4.3) | 3.8(4.3) | 4.0(5.0) | 5.1(4.3) | 5.7(5.5) | 5.9(4.9) | 3.6(3.2) |
| 1998 | 4.9 | 6.0 | 4.4 | 3.8 | 6.2 | 3.6 | 6.1 | 5.8 | 3.4 |
| 1999 | 5.3 | 5.5 | 4.4 | 4.5 | 5.2 | 4.8 | 5.2 | 3.6 | 3.8 |
| 2000 | 5.1 | 5.5 | 4.1 | 3.9 | 6.1 | 4.1 | 6.1 | 5.4 | 4.1 |
| 2001 | 4.0 | 4.8 | 4.9 | 4.2 | 6.7 | 5.4 | 5.7 | 4.6 | 3.2 |
| 2002 | 4.8(4.5) | 5.2(5.2) | 4.4(4.4) | 3.9(4.1) | 5.6(6.0) | 5.8(5.0) | 6.0(5.7) | 6.9(6.1) | 3.6(3.5) |
| 2003 | 4.3 | 4.7 | 4.1 | 4.4 | 5.6 | 4.9 | 5.2 | 6.6 | 3.2 |
| 2004 | 4.4 | 5.6 | 4.6 | 4.0 | 5.9 | 4.8 | 5.7 | 7.0 | 3.4 |
Abbreviations: San Francisco-Oakland (SF-O), State of Connecticut (Connect), State of New Mexico (NM)
Average of annual rates for the indicated period of time
Figure 3.
Three decades (1975−2004) age-adjusted testicular cancer rates among US males (15−49 years) by SEER areas/registries
DISCUSSION
There are some relevant patterns in this trends analysis: 1.) Testicular cancer incidence continues to increase in the US population, albeit the plateau in the 1990s 2.) Incidence rates were highest among whites, intermediate among Asians/Pacific Islanders and American Indian/Alaska native, and lowest among blacks 3.) Blacks demonstrated the highest annual percent changes; indicative of the highest increase in trends in this population, relative to whites and other races 4.) The incidence rates differed by SEER areas and; 5) The lowest rates were reported among age group at diagnosis,15−19 years, and peaked at 30−34 years.
Increase in testicular cancer is observed globally and has been associated with testicular atrophy and decreased spermatogenesis rate. 45 Epidemiologic data postulates and supports cryptorchidism, hypospadias and abnormal spermatogenesis among other factors in testicular carcinogenesis, while environmental toxins acting with estrogenic or anti-androgenic exposure in early fetal development may result in decreased testicular size and impaired spermatogenesis, finally leading to testicular carcinoma. 46
The increasing pattern in testicular neoplasm incidence in United States is an interesting finding albeit the observation that the incidence of this tumor has presented a plateau in recent years in the US population, namely California and our finding of plateau during the 1990s. In this study we observed significant increases in testicular neoplasm in the specific-ages at diagnosis (15−49) and the year of diagnosis, 1975−2004 among all races. Although the testicular cancer incidence rate among blacks is strikingly low compared to whites, the rates fluctuated from 0.3% to 1.4% per 100,000 during this period. However, blacks presented with the highest annual percent change, 2.3% per 100,000, p > 0.05. To our knowledge this paper represents one of the most recent trends study in testicular neoplasm incidence, covering three decades of reported cases (1975−2004). Whereas the incidence rates have fluctuated in the total population, within age-specific strata at diagnosis, race and geographic locale (SEER registries), our data supports significant increasing incidence rates in the three decades of reported cases (1975−2004), and percent change of 71.9 %, with a statistically significant annual percent change of 1.6%, p < 0.05. Our data also support previous findings on increasing testicular cancer trends in the US 6,7, industrialized nations,8 and worldwide. 21,22,45
The examination of the data by race showed differences in rates and distinctive increasing trends across all racial groups, especially whites. The annual percent change was statistically significantly different from zero for all ethnic/racial groups, with blacks demonstrating the highest annual percent change, 2.3%, p<0.05. This result is similar to most published descriptive epidemiologic findings of testicular cancer and racial variation in the United States, suggesting several clues to the determinants of testicular cancer incidence. Since the age at diagnosis is different from most age patterns in malignancies, with testicular germ cell increasing after puberty and reaching its peak incidence at 30 to 34 years (all races and whites) according to our data, in-utero hormonal environment might influence the development of this tumor 23.
The continuing racial disparities in testicular cancer rates may be explained by gestational hormonal differences, genetic susceptibility, environmental pollution exposure and occupational exposure. Furthermore, studies on body mass index (BMI) and testicular cancer etiology are conflicting; while some have observed no clear association, 40,43 others have reported increased risk for testicular cancer with elevated adult BMI, 41 high birth weight, 34 and some have reported increase risk with low BMI. 42 Thus, the association between BMI and testicular cancer remains unclear.
A possible plausible explanation comes from gestational hormonal differences, namely differences in in-utero exposure to testosterone and estrogen, with increased risk of testicular cancer associated with elevated levels of estrogen in-utero.24 Gestational hormonal difference has been evaluated, with testosterone found to be 48% higher in African American women compared to Caucasians counterparts.25 Another study observed maternal serum testosterone levels to be 84% higher and androstenedione to be 52% higher in blacks compared to whites.26 Additionally, a more recent study found maternal serum testosterone and androstenedione to be 69% and 31% higher respectively in blacks compared to whites, 27 suggesting that these hormones may account for the disparity in testicular neoplasm between blacks and whites. There is a possibility that hormonal differences may act in concert with genetic, life style or environmental influences to initiate and promote testicular carcinogenesis. Therefore, the racial disparity in testicular neoplasm remains to be conclusively explained.
Previous studies have found the age at diagnosis of testicular germ cell tumors to be distinct from most tumors. Our study observed the peak incidence at age 30−34 (all races and whites), which is consistent with most findings.3, 15, 28-29 This age distribution is different from that of most malignancies, which normally peaks and increases with advancing age. This distinct age at diagnosis would seem to suggest the role of one single or a few strong risk factors. The problem may be due to the lack of variation in the relevant factors within the studied populations, or to difficulties with exposure characterization. Age-pattern of testicular cancer incidence suggests androgens' role in this carcinogenesis: testicular cancer does not occur in pre-pubertal males, but the incidence increases rapidly after the onset of puberty.
Whereas the incidence of testicular cancer did plateau in the 1990s as confirmed by our analysis, the recent increase in testicular cancer incidence rate among US males may be attributed to influences of risk factors. Remarkably, another possible explanation may be dietary products consumption, a trend that probably started in the forties (1940s) and fifties (1950s).30 The increased incidence of testicular cancer in the past fifty years in Western countries may be associated with the increased consumption of milk and diary products (female sex hormones are present in milk and dairy products). 30, 31 Thus, racial disparities in testicular cancer incidence in our data may be due to the population difference in milk and dairy product consumption, in addition to the variation in maternal serum hormonal levels (androgens and estrogens). Epidemiologic studies including ecologic designs on the racial variation in milk consumption are necessary to assess this correlation. It remains unclear if the observed plateau in the 1990s is associated with increase in early orchidopexy to correct crytorchidism, since undescended testes only accounts for an estimated 10% of testicular cancer risk.18
The general increase in the testicular neoplasm incidence (age-specific, locale and race) observed in our data may be due to variety of factors not examined in this trends study. Increase in surveillance resulting in early detection and asymptomatic cases may be proposed as a possible explanation for the increase in incidence rates. However, this explanation seems implausible given the lack of screening and the symptomatic presentation of testicular cancer at diagnosis. Also, high socio-economic status (SES) has been observed to be associated with increasing risk. 32,33 Given the racial variation in SES in the US population and testicular cancer incidence in our study population, it is possible that certain factors associated with high SES influence lifestyle, which may modulate testicular carcinogenesis. Some studies have indicated a link between increased maternal age and low parity and testicular cancer risk. 34,35 These factors are associated with maternal estrogen elevation at pregnancy, a variable claimed to increase the risk of abnormal gonadal development resulting in testicular cancer. 36,37 Increased maternal age and low parity are observed in the US population. These factors may account for a small fraction of increase in the incidence of testicular cancer. Although it is unclear what the risk factor may be for the variation in the peak age at diagnosis, one might suggest exogenous risk factors that may be associated with the higher prevalence of HIV infection, a risk associated with germ cell testicular neoplasm, mainly seminoma. 38,44
In this analysis, we have shown that the age-adjusted incidence rate of testicular cancer continues to increase among men aged 15−49 years in the US and that there are racial/ethnic variations in rates. These variations may be due to shared environment, maternal serum estrogen and anti-androgen racial variation, HIV infection, dietary patterns, genetic susceptibility, differences in exposure to environmental pollutants/toxins and occupational exposure. In spite of the striking low rate of testicular cancer among blacks, there is a significant increase in annual percent change. These increases and variations in the rates of testicular cancer call for further epidemiologic investigations to assess between sub-population differences and within sub-population trends in the prevalence of candidate risk factors. Finally, the highest annual percent change and unexpected increase in testicular cancer among blacks in the second half of 1990s that have been observed by us and some recent findings39, require further investigation for possible etiologic explanations.
This study has some limitations. First, although we reported the rates by age at diagnosis, birth cohort data (year of birth) could have provided an additional information in terms of rates' determinants. Second, rates based on SEER areas may reflect racial variance in rates. Third, we were unable to show the rates among Hispanics due to the selection of race category that did not sub-classify this racial group. Fourth, the combination of Asian/Pacific Islanders and American Indian/Alaska Natives makes it too broad to define others, rendering this analysis a comparison of blacks versus whites.
In summary, despite these limitations, this study has shown three distinct patterns in testicular incidence trends, early increase in the 1980s, plateaus in the 1990s and increase in 2000s. Secondly, incidence rates continue to vary by race, with black males demonstrating the highest annual percent change, requiring therefore epidemiologic studies to examine possible risk variables including HIV infection prevalence in this shifting paradigm. Further studies may be needed to examine the birth cohort effect (year of birth as population parameter), using three decades data on testicular cancer trends.
ACKNOWLEDGEMENTS
The preparation of this manuscript was facilitated by the National Institute of Mental Health grant number RO1 MH073361-02. The Authors are grateful to the National Cancer Institute, SEER program for the creation and use of this database. The analysis, interpretation and the dissemination of the result of these data remain the sole responsibilities of the authors. We thank Ms. Sandra Pontello for her support in arranging the tables and figures in this manuscript. Also, we are thankful to Jennifer Krueger, Jonathan Brunt and Dr. Emmanuel Monjok for their assistance in preparing of the tables in this manuscript.
Footnotes
COMPETING INTERSTS All authors declare no conflict of interest.
REFERENCES
- 1.National Cancer Institute . SEER*Stat version 6.1.4 SEER cancer incidence public use database, 1973−2002. national cancer institute; Bethesda: 2004. [Google Scholar]
- 2.Jorgensen N, Rajpert-De Meytes E, Graem N, et al. Expression of immunohistochemical markers for testicular in situ by normal human fetal germ cells. Lab. Invest. 1995;72:223–231. [PubMed] [Google Scholar]
- 3.Moller H. Clues to the aetiology of testicular germ cell tumours from descriptive epidemiology. Eur Urol. 1993;23:8–13. doi: 10.1159/000474564. [DOI] [PubMed] [Google Scholar]
- 4.Tamimi R, Adami H. Testicular Cancer. In: Adami H, Hunter D, Trichopoulos D, editors. Textbook of Cancer Epidemiology. Oxford University; New York: 2002. p. 429. [Google Scholar]
- 5.Weir HK, Marrett LD, Moravan V. Trends in the incidence of testicular germ cell cancer in Ontario by histologic subgroup, 1964−1996. CMAJ. 1999;160:201–5. [PMC free article] [PubMed] [Google Scholar]
- 6.McKieman JM, Goluboff ET, Liberson GL, et al. Rising risk of testicular cancer by birth cohort in the United States from 1973−95. J Urol. 1999;162:361–3. [PubMed] [Google Scholar]
- 7.Zheng T, Holford TR, Ma Z, Ward BA, Flannery J, Boyle P. Continuing increase in incidence of germ cell testis cancer in young adults: experience from Connecticut, USA, 1935−1992. Int J Cancer. 1996;65:723–9. doi: 10.1002/(SICI)1097-0215(19960315)65:6<723::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Bergstrom R, Adami HO, Mohner M, et al. Increase in testicular cancer incidence in six European countries: a birth cohort phenomenon. J Natl Cancer Inst. 1996;88:727–33. doi: 10.1093/jnci/88.11.727. [DOI] [PubMed] [Google Scholar]
- 9.Purdue MP, Devesa SS, Sigurdson AJ, McGlynn KA. International patterns and trends in testis cancer incidence. In J Cancer. 2005;115:822–827. doi: 10.1002/ijc.20931. [DOI] [PubMed] [Google Scholar]
- 10.Beutow SA. Epidemiology of testicular cancer. Epidem Rev. 1995;17:433–449. doi: 10.1093/oxfordjournals.epirev.a036202. [DOI] [PubMed] [Google Scholar]
- 11.Kamdar RH, Oliver RTD, Othieno-Abinya N, Gallager CJ, Slevin ML. Geographical epidemiology of ovarian and testicular germ cells cancers. Br J cancer. 1998;78:1401–3. doi: 10.1038/bjc.1998.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolonel LN, Ross RK, Thomas DB, Thompson DJ. Epidemiology of testicular cancer in the pacific basin. Natl Cancer Inst Monogr. 1982;62:157–160. [PubMed] [Google Scholar]
- 13.Talerman A, Kaalen JG, Fokkens W. Rural preponderance of testicular neoplasms. Br J Cancer. 1974;29:176–8. doi: 10.1038/bjc.1974.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sonneveld DJ, Schaapveld M, Sleijfer DT, Graff WT, Sijmons RH, Koops HS, et al. Geographic clustering of testicular cancer incidence in the northern part of The Netherlands. Br J cancer. 1999;81:1262–7. doi: 10.1038/sj.bjc.6690839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schouten LJ, Meijer H, Huveneers JA, Kiemeney LA. Urban-rural differences in cancer incidence in The Netherlands 1989−1991. Int J epidemiol. 1996;25:729–36. doi: 10.1093/ije/25.4.729. [DOI] [PubMed] [Google Scholar]
- 16.Ross RK, McCurtis JW, Henderson BE, Menck HR, Mack TM, Martin SP. Descriptive epidemiology of testicular and prostate cancer in Los Angeles. Br J Cancer. 1979;39:284–92. doi: 10.1038/bjc.1979.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen MM, Ellison LM. Testicular cancer pattern in Asian-Americans males: An opportunity for public health education to impact outcomes. Urology. 2005;66:606–609. doi: 10.1016/j.urology.2005.03.069. [DOI] [PubMed] [Google Scholar]
- 18.Pharris-Cirej ND, Good LS, Weiss NS. Incidence of testicular cancer in the United States : Has the epidemic begun to abate? Am J Epidemiol. 1999;150:45–46. doi: 10.1093/oxfordjournals.aje.a009916. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results. SEER*Stat Database: Incidence – SEER 9 Registries Limited-Use, November 2006 Sub (1973−2004), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission [http://www.seer.cancer.gov/data]
- 20.Coleman MP, Esteve J, Damiecki P. Trends in cancer incidence and mortality. IARC Sci. Publ. 1993;121:521–542. doi: 10.3109/9780415874984-2. [DOI] [PubMed] [Google Scholar]
- 21.Schottenfeld D. Testicular cancer. In: Schottenfeld D, Fraumeni JE, Jr., editors. Cancer epidemiology and prevention. WB Saunders; Philadelphia: 1996. pp. 1207–1219. [Google Scholar]
- 22.Hemderson BE, Ross RK, Pick MC, Depue RH. Epidemiology of testis cancer. In: Skinner D, editor. Urological cancer. Grune & Stratton; New York: 1983. p. 237. [Google Scholar]
- 23.Wanderas EH, Grotmol T, Fossa SD, Tretli S. Maternal health and pre and perinatal characteristics in the etiology of testicular cancer: a prospective population and register-based study on Norwegian males born between 1967 and 1995. Cancer Causes Control. 1998;14:475–86. doi: 10.1023/a:1008857702380. [DOI] [PubMed] [Google Scholar]
- 24.Henderson JE, Bernstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of Blacks and Whites: potential effect on male offspring. Br J Cancer. 1988;57:216–8. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Troisi R, Potischman N, Roberts JM, Siiteri P, Hoover RN. Associations of maternal and umbilical cord hormone concentrations with maternal , gestational and neonatal factors (United States). Cancer causes Control. 2003;14:347–55. doi: 10.1023/a:1023934518975. [DOI] [PubMed] [Google Scholar]
- 26.Potischam N, Triosi R, Thadhani R, Hoover R, Dodd K, Davis WW, et al. Pregnancy hormone concentrations across ethnic groups: Implications for later cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1514–20. doi: 10.1158/1055-9965.EPI-04-0869. [DOI] [PubMed] [Google Scholar]
- 27.Liu S, Wen SW, Mao Y, Mery L, Rouleau J. Birth cohor effects underlying the increasing testicular cancer incidence in Canada. Can J Public Health. 1999:90176–80. doi: 10.1007/BF03404502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller H. Trends in incidence of testicular cancer and prostate cancer in Denmark. Human Reproduction. 2001;16:1007–1011. doi: 10.1093/humrep/16.5.1007. [DOI] [PubMed] [Google Scholar]
- 29.Ganmaa D, Wang PY, Qin LQ, et al. Is milk responsible for male reproductive disorders? Med Hypoth. 2001;57:510–4. doi: 10.1054/mehy.2001.1380. [DOI] [PubMed] [Google Scholar]
- 30.Davis TW, Palmer CR, Ruja E, et al. Adolescent milk, dairy product and fruit consumption and testicular cancer. Br J cancer. 1996;74:657–60. doi: 10.1038/bjc.1996.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swerdlow AJ, Skeet RG. Occupational associations of testicular cancer in southease England. Br J Ind Med. 1988;45:225–30. doi: 10.1136/oem.45.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce N, Sheppard RA, Howard JK, Fraser J, Lilley BM. Time trends and occupational differences in cancer of the testis in New Zealand. Cancer. 1987;59:1677–82. doi: 10.1002/1097-0142(19870501)59:9<1677::aid-cncr2820590926>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.Moller H, Skakkebaek NE. Testicular cancer and cryptorchidism in relation to prenatal factors: case control studies in Denmark. Cancer Causes Control. 1997;8:904–12. doi: 10.1023/a:1018472530653. [DOI] [PubMed] [Google Scholar]
- 34.Panagiotopoulou K, Katsouyanni K, Petridou E, Garas Y, Tzonou A, Trichopoulos D. Maternal age , parity, and preganancy estrogens. Cancer Causes Control. 1990;1:119–24. doi: 10.1007/BF00053162. [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow AJ, Huttly SR, Smith PG. Prenatal and familial associations of testicular cancer. Br J Cancer 1987. 1987;55:571–7. doi: 10.1038/bjc.1987.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trichopoulos D, Cole P, Brown JB, Goldman MB, MacMahon B. Estrogen profiles of primiparous women inAthens, Greece. J. natl cancer Inst. 1980;65:43–6. [PubMed] [Google Scholar]
- 37.Frish M, Bigger RJ, Engles EA, Goedert JJ. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–45. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 38.McGlynn KA, Devesa SS, Graubard BI, Castle PE. Increasing Incidence of Testicular Germ Cell Tumors Among Black Men in the United States. J Clin Oncol. 2005;23:5757–5761. doi: 10.1200/JCO.2005.08.227. [DOI] [PubMed] [Google Scholar]
- 39.Richiardi L, Askling J, Granath F, Akre O. Body size at Birth and adulthood and the Risk for Germ-cell Testicular Cancer. Cancer Epidemiol Biomarkers Prev. 2003;12:669–673. [PubMed] [Google Scholar]
- 40.Dieckmann KP, Pichlmeier U. Is testicular cancer related to body size? Eur Urol. 2002;42:564–569. doi: 10.1016/s0302-2838(02)00467-0. 2002. [DOI] [PubMed] [Google Scholar]
- 41.Petridou E, Roukas KI, Dessypris N, Aravantinos G, Bafaloukos D, Efraimidis A, et al. Baldness and other correlates of sex hormones in relation to testicular cancer. Int J. cancer. 1997;71:982–985. doi: 10.1002/(sici)1097-0215(19970611)71:6<982::aid-ijc13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Akre O, Ekbom A, Sparen P, Tretli S. Body size and testicular cancer. J Natl Cancer Inst. 2000;92:1093–1096. doi: 10.1093/jnci/92.13.1093. [DOI] [PubMed] [Google Scholar]
- 43.Powles T, Bower M, Daugaard G, et al. Multicenter study of human immunodeficiency virus-related germ cell tumors. J Clin Oncol. 2003;21:1922–1927. doi: 10.1200/JCO.2003.09.107. [DOI] [PubMed] [Google Scholar]
- 44.Pajarinen J, Laippala P, Penttila A, et al. Incidence of disorders of spermatogenesis in middle aged Finnish men, 1981−91: two necropsy series. BMJ. 1997;314:13–18. doi: 10.1136/bmj.314.7073.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klotz LH. Why is the rate of testicular cancer increasing? CMAJ. 1999;160:213–214. [PMC free article] [PubMed] [Google Scholar]